ID: 1
InChIKey: TZBJGXHYKVUXJN-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment several
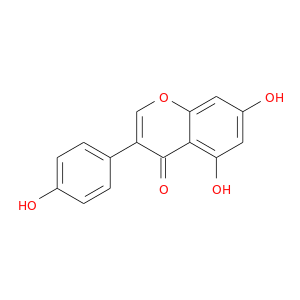
CID is 5280961
synonyms found at PubChem are:
genistein, 446-72-0, Prunetol, Genisteol, 4',5,7-Trihydroxyisoflavone, Genisterin, Sophoricol, 5,7,4'-Trihydroxyisoflavone, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one, Bonistein, Genestein, Differenol A, NPI 031L, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, C.I. 75610, SIPI 807-1, 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one, UNII-DH2M523P0H, NSC 36586, 4,5,7-Trihydroxyiso-flavone, Lactoferrin-genistein, CCRIS 7675, 4',5, 7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone, NSC36586, CHEMBL44, EINECS 207-174-9, IN1327, BRN 0263823, PTI G4660 (Genistein), ISOFLAVONE, 4',5,7-TRIHYDROXY-, MLS000738127, DH2M523P0H, STO514, CHEBI:28088, PTI-G4660, SIPI-9764-I, TZBJGXHYKVUXJN-UHFFFAOYSA-N, 4′,5,7-Trihydroxyisoflavone, TNP00151, NSC-36586, GENISTEIN (ENDOCRINE DISRUPTER), GEN, NCGC00015479-09, DSSTox_CID_2308, G 6649, G10000, DSSTox_RID_76542, DSSTox_GSID_22308, ENDOCRINE DISRUPTOR (GENISTEIN) (SEE ALSO GENISTEIN (446-72-0)), CAS-446-72-0, SR-01000075498, HSDB 7475, PTI G4660, 3kgt, 3kgu, 4&prime, Genistein, 8, Genistein,(S), PTI-G 4660, Genistein (flavonoid), PubChem9852, Spectrum_000320, Tocris-1110, 1x7r, 2qa8, Genistein 85% HPLC, SpecPlus_000305, AC1NQXT4, Spectrum2_000638, Spectrum3_000678, Spectrum4_001543, Spectrum5_000106, Lopac-G-6649, 4',7-Trihydroxyisoflavone, D0L4FS, MolMap_000022, UPCMLD-DP096, 4,5,7-Trihydroxyisoflavone, 4,6,7-Trihydroxyisoflavone, Isoflavone,5,7-trihydroxy-, Lopac0_000520, Oprea1_224620, Oprea1_437815, SCHEMBL19166, BSPBio_002375, KBioGR_002006, KBioGR_002564, KBioSS_000800, KBioSS_002573, SPECTRUM210296, 5-18-04-00594 (Beilstein Handbook Reference), BIDD:ER0113, DivK1c_006401, Genistein, analytical standard, SPBio_000636, 4',5,7-Trihydroxy isoflavone, 4',5,7-trihydroxy-Isoflavone, GTPL2826, MEGxp0_000568, 4,5,7-Trihydroxy Iso-Flavone, DTXSID5022308, UPCMLD-DP096:001, ACon1_001065, BDBM19459, cid_5280961, KBio1_001345, KBio2_000800, KBio2_002564, KBio2_003368, KBio2_005132, KBio2_005936, KBio2_007700, KBio3_001595, KBio3_003042, AOB5073, CHEBI: 28088, cMAP_000086, MolPort-000-003-911, Bio1_000445, Bio1_000934, Bio1_001423, HMS2271K09, HMS3261H21, HMS3267K14, HMS3428M01, HMS3649B22, HMS3654D17, ACT05962, ALBB-015886, BCP07581, KS-00000MH8, Prunetol solution, 20 mM in DMSO, Tox21_110161, Tox21_201428, Tox21_300585, Tox21_500520, AC-472, BBL010484, CCG-38551, CG-009, Genistein solution, 20 mM in DMSO, LMPK12050218, MFCD00016952, s1342, SBB066115, STK801619, ZINC18825330, AKOS001590147, Tox21_110161_1, CS-1534, DB01645, KS-5128, LP00520, LS-1266, MCULE-4857649752, RL03694, RP29616, SMP1_000133, Genistein; 4',5,7-Trihydroxyisoflavone, NCGC00015479-01, NCGC00015479-02, NCGC00015479-04, NCGC00015479-05, NCGC00015479-06, NCGC00015479-07, NCGC00015479-08, NCGC00015479-10, NCGC00015479-11, NCGC00015479-12, NCGC00015479-13, NCGC00015479-14, NCGC00015479-15, NCGC00015479-16, NCGC00015479-17, NCGC00015479-18, NCGC00015479-19, NCGC00015479-20, NCGC00025005-01, NCGC00025005-02, NCGC00025005-03, NCGC00025005-04, NCGC00025005-05, NCGC00025005-06, NCGC00025005-07, NCGC00169711-01, NCGC00169711-02, NCGC00254275-01, NCGC00258979-01, NCGC00261205-01, 690224-00-1, AJ-70669, AN-15821, BC200563, HY-14596, KB-52241, NCI60_003369, SC-04581, SMR000112580, ST056352, AB1004490, EU-0100520, FT-0603395, G0272, N1861, Genistein, disposable screening library format, 46G720, C06563, G-2535, Genistein, synthetic, >=98% (HPLC), powder, J10015, K00046, S-7751, US8552057, 2, AB00052696_09, AB00052696_12, A826657, Genistein, primary pharmaceutical reference standard, I06-0431, Q-100484, SR-01000075498-1, SR-01000075498-3, SR-01000075498-6, 3-(4-hydroxyphenyl)-5,7-bis(oxidanyl)chromen-4-one, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone, BRD-K43797669-001-02-3, BRD-K43797669-001-03-1, BRD-K43797669-001-10-6, Genistein, from Glycine max (soybean), ~98% (HPLC), SR-01000075498-10, 5,7-dihydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one, F0001-2388, 4H-1-Benzopyran-4-one,7-dihydroxy-3-(4-hydroxyphenyl)-, UNII-71B37NR06D component TZBJGXHYKVUXJN-UHFFFAOYSA-N, Genistein, United States Pharmacopeia (USP) Reference Standard, Genistein, Pharmaceutical Secondary Standard; Certified Reference Material, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
ID: 121
InChIKey: AFWKBSMFXWNGRE-ONEGZZNKSA-N SMILES: CC(=O)/C=C/C1=CC(=C(C=C1)O)OC
biological descriptors:
CFTR relevance: CFTR activatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
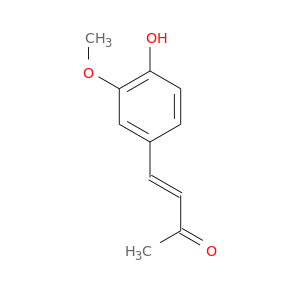
CID is 5354238
synonyms found at PubChem are:
Dehydrozingerone, 1080-12-2, Feruloylmethane, 4-(4-Hydroxy-3-methoxyphenyl)-3-buten-2-one, 4-(4-hydroxy-3-methoxyphenyl)but-3-en-2-one, Vanillalacetone, Dehydro(O)-paradol, Vanillylidene acetone, MHSK, Dehydrogingerone, Vanylidenacetone, 3-BUTEN-2-ONE, 4-(4-HYDROXY-3-METHOXYPHENYL)-, (O)-Paradol, dehydro-, Vanillidene acetone, 3-Methoxy-4-hydroxybenzalacetone, (O)-Dehydroparadol, 4-Hydroxy-3-methoxystyryl methyl ketone, (E)-4-(4-hydroxy-3-methoxyphenyl)but-3-en-2-one, NSC 4019, NSC 5316, UNII-8CJX5I27B7, Vanillylideneacetone, 4-Hydroxy-3-methoxybenzylideneacetone, NSC 26613, NSC 44708, (0)-Dehydroparadol, FEMA No. 3738, Methyl-3-methoxy-4-hydroxy styryl ketone, NSC-4019, NSC-5316, EINECS 214-096-9, NSC 45411, NSC-44708, NSC-45411, BRN 2049660, Methyl-3-methoxy-4-hydroxystyryl ketone, AI3-01934, 8CJX5I27B7, CHEMBL106509, 22214-42-2, CHEBI:81361, Vanillylidenacetone, AFWKBSMFXWNGRE-ONEGZZNKSA-N, [0]-Dehydroparadol, (3E)-4-(4-Hydroxy-3-methoxyphenyl)but-3-en-2-one, [0]-Paradol, dehydro-, dehydrozingerol, AE-562/43459152, W-200772, (E)-4-(4-hydroxy-3-methoxy-phenyl)but-3-en-2-one, C11H12O3, 4-hydroxy-3-methoxybenzalacetone, MMHSK, 3-Buten-2-one,4-(4-hydroxy-3-methoxyphenyl)-, (E)-2-Methoxy-4-(3-oxo-1-butenyl)phenol, D05GRI, AC1NS57G, 2-08-00-00326 (Beilstein Handbook Reference), SCHEMBL498223, ZINC8046, DTXSID2061480, Vanillylidenacetone, >=98.5%, 3-Buten-2-one, 4-(4-hydroxy-3-methoxyphenyl)-, (E)-, NSC4019, NSC5316, MolPort-001-823-649, NSC26613, NSC44708, NSC45411, BDBM50195679, MFCD00012210, NSC-26613, AKOS004909556, DS-4905, FCH5479239, Vanillylidenacetone, analytical standard, AJ-08309, AK135171, AN-19685, BBV-38737560, LS-47310, ZB000663, AX8090618, KB-187080, TC-104629, ST24042954, C17840, 4-(4-Hydroxy-3-methoxy-phenyl)-but-3-en-2-one, 4-(4-Hydroxy-3-methoxyphenyl)-3-buten-2-one #, C-49429, I14-44563, (E)-4-(4-Hydroxy-3-methoxy-phenyl)-but-3-en-2-one
ID: 2
InChIKey: VFLDPWHFBUODDF-FCXRPNKRSA-N SMILES: COC1=C(C=CC(=C1)/C=C/C(=O)CC(=O)/C=C/C2=CC(=C(C=C2)O)OC)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function likely enhances CFTR function
Order of interaction unknown
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
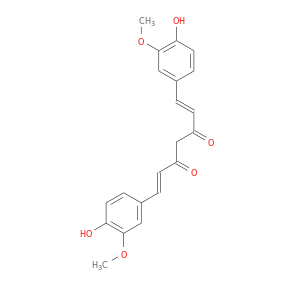
CID is 969516
synonyms found at PubChem are:
curcumin, 458-37-7, Diferuloylmethane, Natural yellow 3, Turmeric yellow, Curcuma, Turmeric, Kacha haldi, Gelbwurz, Indian saffron, Curcumin I, Souchet, Haidr, Halad, Haldar, Halud, Merita earth, Terra Merita, Yellow Ginger, Yellow Root, Safran d'Inde, Yo-Kin, Golden seal, Orange Root, C.I. Natural Yellow 3, Curcumine, Hydrastis, Indian turmeric, Yellow puccoon, Curcuma oil, Diferaloylmethane, Turmeric extract, Oils, curcuma, Kurkumin [Czech], (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione, Tumeric yellow, Turmeric oil, Oil of turmeric, CI Natural Yellow 3, Turmeric oleoresin, Curcuma longa oils, Zlut prirodni 3 [Czech], Cucurmin, Kurkumin, C.I. 75300, Tumeric oleoresin, Zlut prirodni 3, Turmeric root oil, Turmeric, oleoresin, Curcuma oil (Curcuma longa), curouma, E 100, Curcurmin, Diferulylmethane, kachs haldi, safra d'inde, Turmeric oil (Curcuma longa L.), Curcuma longa l. root oil, 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, NSC32982, UNII-IT942ZTH98, NSC 32982, Tu rmeric root oil, Turmeric (>98% curcurmin), 98% curcurmin), FEMA No. 3085, FEMA No. 3086, CCRIS 3257, CCRIS 5804, CHEBI:3962, 1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione, MLS000069631, Turmeric oleoresin (79%-85% curcumin), Curcuma longa l. oil, HSDB 4334, Turmeric root oleoresin, 1,5-Di(vanillyliden)acetylaceton, NCI-C61325, C21H20O6, 1,5-Divanillyliden-2,4-pentandion, EINECS 207-280-5, Turmeric extract (Curcuma longa L.), 8024-37-1, 1,9-Bis(4-hydroxy-3-methoxyphenyl)-2,7-nonadiene-4,6-dione, CHEMBL140, Curcuma longa l. oleoresin, NSC 687842, BRN 2306965, 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (E,E)-, CI 75300, SMR000058237, 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (1E,6E)-, IT942ZTH98, Curcuma longa l. root oleoresin, VFLDPWHFBUODDF-FCXRPNKRSA-N, (E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, NSC-32982, NSC687842, NCGC00017159-05, DSSTox_CID_1421, (1E,6E)-1,7-bis(4-hydroxy-3-methoxy-phenyl)hepta-1,6-diene-3,5-dione, (1E,6E)-1,7-bis[4-hydroxy-3-(methyloxy)phenyl]hepta-1,6-diene-3,5-dione, DSSTox_RID_78861, DSSTox_GSID_31077, CU-01000001305-2, CURCUMIN (SEE ALSO TUMERIC, OLEORESIN (10105-J)), 2,7-Nonadiene-4,6-dione, 1,9-bis(4-hydroxy-3-methoxyphenyl)-, Oils, galangal, PREVENTION 4 (CURCUMIN) (SEE ALSO TUMERIC, OLEORESIN (10105-J)), TURMERIC, OLEORESIN (CURCUMIN) (SEE ALSO CURCURMIN (458-37-7)), Yellow, Turmeric, 91884-86-5, CAS-458-37-7, 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, SR-01000000149, diferuloylmethan, E 100 (Dye), Haldar, Souchet, Natural Yellow3, Curcumin,(S), Curcumin (Natural), trans,trans-Curcumin, Opera_ID_1627, D07SDQ, D0H8LC, AC1LJ6T6, SCHEMBL8440, SCHEMBL8441, Curcumin, analytical standard, 4-08-00-03697 (Beilstein Handbook Reference), MLS001148449, BIDD:ER0479, turmeric root oil CO2 extract, AC1Q46A0, cid_969516, GTPL7000, turmeric root oil hydrodistilled, DTXSID8031077, SCHEMBL13521974, BDBM29532, cid_5281767, BIC8500, cMAP_000052, MolPort-001-763-682, HMS2233K04, HMS3649K06, ZINC899824, BB_NC-01422, BCP04695, HY-N0005, ZX-AT003872, Tox21_110803, Tox21_111505, Tox21_201116, BBL027711, BDBM50067040, BDBM50140172, BG0601, CC0179, CCG-36020, CCG-36107, GP8291, LS-125, MFCD00008365, SBB006495, STL371943, AKOS001305497, BCP9000557, CS-1490, curcuma longa l. root oil CO2 extract, DB11672, LS-2189, NSC-687842, OR24598, RTR-032605, curcuma longa l. root oil hydrodistilled, NCGC00017159-04, NCGC00017159-06, NCGC00017159-07, NCGC00017159-09, NCGC00017159-10, NCGC00017159-11, NCGC00017159-12, NCGC00023332-03, NCGC00023332-04, NCGC00023332-05, NCGC00258668-01, AC-24238, AN-23454, BC678109, M212, SC-17381, ST055629, BCP0726000035, DB-002681, KB-251224, TR-032605, WLN: 1OR BQ E1U1V1V1U1R DQ CO1, N1839, 1,3-Di(3-methoxy-4-hydroxystyryl)propanedial, 1790-EP2305629A1, 1790-EP2308861A1, J10108, K00009, W-5038, Curcumin, from Curcuma longa (Turmeric), powder, 458C377, Curcumin, primary pharmaceutical reference standard, 1,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, I06-0010, I06-2497, SR-01000000149-2, SR-01000000149-5, BRD-K07572174-001-02-2, BRD-K07572174-001-19-6, BRD-K07572174-001-22-0, I14-19358, Curcumin, >=94% (curcuminoid content), >=80% (Curcumin), 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)1,6-heptadiene-3,5-dione, 1,7-DI(4-HYDROXY-3-METHOXYPHENYL)HEPTA-1,6-DIENE-3, Curcumin, matrix substance for MALDI-MS, >=99.5% (HPLC), Curcumin, United States Pharmacopeia (USP) Reference Standard, 1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione, 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), (1E,6E)-1,7-bis(3-methoxy-4-oxidanyl-phenyl)hepta-1,6-diene-3,5-dione, (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione #, (1E,6E)-1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione, (1Z,6E)-1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione, 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, (E,E)-, 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one, 5-Hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one, Curcumin; 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, (1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one, 15845-47-3, 33171-04-9, 73729-23-4, 79257-48-0, Curcumin solution, ~0.1 % (w/v) (in ethanol with 2M HCl (99:1 v/v)), for TLC derivatization
ID: 11
InChIKey: WBSMIPAMAXNXFS-UHFFFAOYSA-N SMILES: C1=CC=C(C=C1)CCCNC2=C(C=C(C=C2)[N+](=O)[O-])C(=O)O
biological descriptors:
CFTR relevance: CFTR activatorCategory:
Influence on CFTR function inconsistent assignment
Order of interaction binds to CFTR
subcellular compartment Apical membrane & subapical compartment
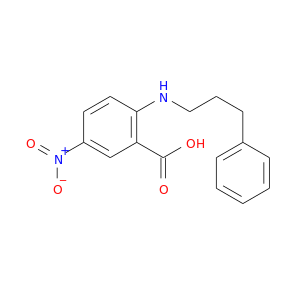
CID is 4549
synonyms found at PubChem are:
NPPB, 5-Nitro-2-(3-phenylpropylamino)benzoic acid, 107254-86-4, UNII-3A35O9G3YZ, 5-nitro-2-[(3-phenylpropyl)amino]benzoic acid, 3A35O9G3YZ, CHEBI:34457, HOE 144, HOE-144, Benzoic acid,5-nitro-2-[(3-phenylpropyl)amino]-, Benzoic acid, 5-nitro-2-((3-phenylpropyl)amino)-, IN1196, 5-NITRO-2-(3-PHENYLPROPYLAMINO)-BENZOIC ACID, 5-NITRO-2-PHENYLPROPYLAMINOBENZOIC ACID [NPPB], 5-Nitro-2-(3-phenylpropylamino)benzoic Acid (NPPB), SR-01000075336, Spectrum_001814, Tocris-0593, ACMC-20c9kv, AC1L1IEV, Spectrum2_001477, Spectrum3_001518, Spectrum4_000338, Spectrum5_001244, Lopac-N-4779, (NPPB), D08DQL, D0HW9P, UPCMLD-DP143, CBiol_001838, Lopac0_000857, BSPBio_001423, BSPBio_003195, KBioGR_000715, KBioSS_002311, MLS000859983, DivK1c_000619, SCHEMBL159244, SPBio_001433, AC1Q20U0, GTPL4270, CHEMBL1256759, UPCMLD-DP143:001, UPCMLD-DP143:002, CTK4A5098, HMS501O21, KBio1_000619, KBio2_002309, KBio2_004877, KBio2_007445, KBio3_002695, DTXSID90147978, MolPort-003-959-000, NINDS_000619, WBSMIPAMAXNXFS-UHFFFAOYSA-N, Bio1_000124, Bio1_000613, Bio1_001102, HMS1791H05, HMS1989H05, HMS2235P03, HMS3262L15, HMS3266O16, HMS3373B17, HMS3402H05, ZINC3873822, Tox21_500857, BN0393, CCG-39187, MFCD00153851, AKOS024458574, CS-8179, LP00857, IDI1_000619, NCGC00015740-01, NCGC00015740-02, NCGC00015740-03, NCGC00015740-04, NCGC00015740-05, NCGC00015740-06, NCGC00015740-07, NCGC00015740-08, NCGC00015740-09, NCGC00015740-10, NCGC00024671-01, NCGC00024671-02, NCGC00024671-03, NCGC00024671-04, NCGC00024671-05, NCGC00024671-06, NCGC00024671-07, NCGC00024671-08, NCGC00261542-01, SMR000326842, Brit J Pharmacol 117: 175 (1996), HY-101012, LS-186884, LS-187538, RT-010388, 2-PHENPROPYLAMINO-5-NITROBENZOIC ACID, 5 nitro 2 (3 phenylpropylamino)benzoic acid, B6367, EU-0100857, X6841, 5-nitro-2-(3-phenylpropylamino) benzoic acid, 5-Nitro-2-(3'-phenylpropyl-amino)benzoic acid, 5-Nitro-2-(3-phenyl-propylamino)-benzoic acid, C13705, N 4779, J-001745, SR-01000075336-1, SR-01000075336-3, 5-Nitro-2-(3-phenylpropylamino)benzoic acid, >=98%, BRD-K89272762-001-02-8, BRD-K89272762-001-04-4, BRD-K89272762-001-07-7