ID: 5
InChIKey: LUKBXSAWLPMMSZ-OWOJBTEDSA-N SMILES: C1=CC(=CC=C1/C=C/C2=CC(=CC(=C2)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment Apical membrane & subapical compartment
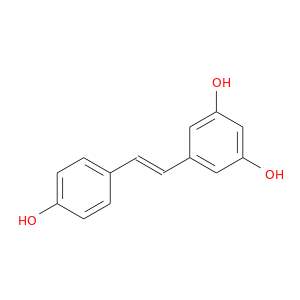
CID is 445154
synonyms found at PubChem are:
resveratrol, 501-36-0, trans-resveratrol, 3,4',5-Trihydroxystilbene, (E)-5-(4-Hydroxystyryl)benzene-1,3-diol, 3,5,4'-Trihydroxystilbene, (E)-resveratrol, 3,4',5-Stilbenetriol, Resvida, 3,4',5-Trihydroxy-trans-stilbene, 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol, SRT-501, SRT501, Resveratol, SRT 501, 3,5,4'-Trihydroxy-trans-stilbene, 5-[(E)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol, 5-[(1E)-2-(4-Hydroxyphenyl)ethenyl]-1,3-benzenediol, 3,4',5-trihydroxy-stilbene, Resveratrol, natural, UNII-Q369O8926L, CHEBI:45713, trans-3,4',5-trihydroxystilbene, (E)-5-(p-Hydroxystyryl)resorcinol, trans-1,2-(3,4',5-Trihydroxydiphenyl)ethylene, 5-[2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol, CHEMBL165, NSC 327430, (E)-5-[2-(4-hydroxyphenyl)ethenyl]-1,3-benzendiol, MLS000069735, 1,3-Benzenediol, 5-(2-(4-hydroxyphenyl)ethenyl)-, (E)-, LUKBXSAWLPMMSZ-OWOJBTEDSA-N, KUC104385N, PREVENTION 8 (RESVERATROL), C14H12O3, trans-3,4',5 - trihydroxystilbene, NSC327430, KSC-10-164, Q369O8926L, 5-((1E)-2-(4-Hydroxyphenyl)ethenyl)-1,3-benzenediol, RM-1812, trans-3,4′,5-Trihydroxystilbene, SMR000058206, R 5010, DSSTox_CID_11980, DSSTox_RID_78898, DSSTox_GSID_31980, CU-01000001503-3, (E)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol, (E)-5-[2-(4-Hydroxyphenyl)ethenyl]-1,3-benzenediol, 1,3-Benzenediol, 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]-, MFCD00133799, STL, SR-01000000163, 5-[(1E)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol, CHEBI:27881, CCRIS 8952, HSDB 7571, 3fts, 4jaz, 4qer, Resveratrol, E-, Resveratrol,(S), Stilbene, 2f, TaxusChinensisiRehd, NCGC00015894-02, CAS-501-36-0, Prestwick_619, Trans-3,4&prime, Resveratrol, trans-, Resveratrol, synthetic, Opera_ID_586, AC1L9HIC, Prestwick2_000508, Prestwick3_000508, Spectrum5_000552, D0U3EP, Lopac0_001111, REGID_for_CID_6240, SCHEMBL19425, BSPBio_000435, BSPBio_001114, BSPBio_003461, MLS001055357, MLS001076538, MLS001424228, MLS002207121, MLS002222231, ARONIS24568, SPECTRUM1502223, BPBio1_000479, cid_445154, GTPL8741, SGCUT00007, trans-Stilbene-3,4',5-triol, ZINC6787, Resveratrol, analytical standard, DTXSID4031980, REGID_for_CID_445154, BDBM23926, Resveratrol, >=99% (HPLC), BBC/741, BIK9013, 2l98, MolPort-002-499-801, BCPP000091, HMS1362H15, HMS1569F17, HMS1792H15, HMS1921N04, HMS1990H15, HMS2052I09, HMS2096F17, HMS2232A18, HMS3263O04, HMS3403H15, HMS3649A20, ACT09778, BCP01416, to_000079, ZX-AS004941, ZX-AT013797, Tox21_110257, Tox21_201374, Tox21_303376, Tox21_501111, ABP000376, AC-727, AN-865, BBL028252, BS0159, CCG-38874, CR-003, GP2549, GP5884, LMPK13090005, s1396, SBB055452, STL146386, AKOS005720936, Tox21_110257_1, ACN-034773, API0000480, CS-1050, DB02709, KS-5047, LP01111, LS-2146, MCULE-5678456463, NC00349, NSC-327430, OR46018, RP17549, SDCCGMLS-0002998.P003, IDI1_002152, Resveratrol solution, 100 mM in DMSO, NCGC00017352-05, NCGC00017352-06, NCGC00017352-07, NCGC00017352-08, NCGC00017352-09, NCGC00017352-10, NCGC00017352-11, NCGC00017352-12, NCGC00017352-13, NCGC00017352-14, NCGC00017352-15, NCGC00017352-16, NCGC00017352-17, NCGC00017352-18, NCGC00017352-19, NCGC00017352-24, NCGC00024003-00, NCGC00024003-04, NCGC00024003-05, NCGC00024003-06, NCGC00024003-07, NCGC00024003-08, NCGC00024003-09, NCGC00024003-10, NCGC00024003-11, NCGC00024003-12, NCGC00024003-13, NCGC00024003-14, NCGC00257465-01, NCGC00258925-01, NCGC00261796-01, 375823-41-9, 4CN-0696, AJ-08292, AK-39118, AS-12413, BC202036, BR-39118, CC-34242, CJ-00111, CPD000058206, HY-16561, KB-02515, SAM001246888, SC-11924, ST057251, SY014849, ZB000650, AB0006623, AX8004672, ST2408097, TL8003323, EU-0101111, FT-0082623, N1848, R0071, Resveratrol, Vetec(TM) reagent grade, 98%, 01R360, C03582, J10118, N88795, 5-[2-(4-hydroxyphenyl)vinyl]-1,3-benzenediol, AB00052942-29, AB00052942_31, A827984, 5-[(E)-2-(4-Hydroxyphenyl)vinyl]-1,3-benzoldiol, I06-0437, SR-01000000163-3, SR-01000000163-4, SR-01000000163-9, 5-[(E)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol, 5-[(E)-2-(4-Hydroxyphenyl)ethenyl]benzol-1,3-diol, 5-[(E)-2-(4-Hydroxyphenyl)vinyl]-1,3-benzenediol, 5[(E)-2-(4-Hydroxyphenyl)-vinyl]benzene 1,3-diol, BRD-K25591257-001-01-2, BRD-K80738081-001-06-2, BRD-K80738081-001-07-0, BRD-K80738081-001-09-6, BRD-K80738081-001-10-4, BRD-K80738081-001-23-7, SR-01000000163-10, SR-01000000163-11, SR-01000000163-16, (E)-1-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)ethene, (E)1-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)ethene, 5-[(1E)-2-(4-Hydroxyphenyl)ethenyl]-1,3,benzenediol, Resveratrol, certified reference material, TraceCERT(R), 1,3-Benzenediol, 5-[(E)-2-(4-hydroxyphenyl)ethenyl]-, Resveratrol, European Pharmacopoeia (EP) Reference Standard, 533C1DA0-4104-42B5-9D32-9265F40857E4, trans-Resveratrol, United States Pharmacopeia (USP) Reference Standard, 3,4',5-Trihydroxy-trans-stilbene 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol, (E)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol(E)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol, InChI=1/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1, trans-3,5,4'-Trihydroxystilbene3,4',5-Stilbenetrioltrans-Resveratrol(E)-5-(p-Hydroxystyryl)resorcinol(E)-5-(4-hydroxystyryl)benzene-1,3-diol
ID: 17
InChIKey: LUKBXSAWLPMMSZ-UPHRSURJSA-N SMILES: C1=CC(=CC=C1/C=C\C2=CC(=CC(=C2)O)O)O
biological descriptors:
CFTR relevance: CFTR potentiatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment Apical membrane & subapical compartment
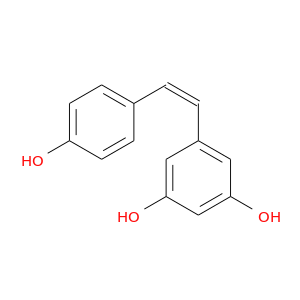
CID is 1548910
synonyms found at PubChem are:
cis-resveratrol, (Z)-resveratrol, 61434-67-1, Cis resveratrol, Resveratrol, (Z)-, UNII-AUA0K06FSB, cis-3,4',5-trihydroxystilbene, AUA0K06FSB, CHEMBL87333, CHEBI:36002, 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol, 5-[(Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol, 5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol, 1,3-Benzenediol, 5-((1Z)-2-(4-hydroxyphenyl)ethenyl)-, Resveratrol (Z)-form [MI], Z-Resveratrol, Resveratrol, Z-, NCGC00015894-02, CAS-501-36-0, Tocris-1418, AC1LU7HY, Lopac-R-5010, cis-3,4,5-Trihydroxystilbene, SCHEMBL1931746, cis-3,5,4'-trihydroxystilbene, LUKBXSAWLPMMSZ-UPHRSURJSA-, (Z)-3,4',5-trihydroxystilbene, (Z)-3,5,4'-trihydroxystilbene, 4q93, MolPort-003-850-143, TNP00294, 1684AH, BDBM50131698, ZINC12353732, AKOS025395422, NCGC00015894-01, NCGC00017352-01, NCGC00017352-02, NCGC00017352-03, NCGC00017352-04, NCGC00024003-02, AC-24235, CJ-13797, DB-072954, 434C671, I14-7425, Z-5-[2-(4-Hydroxyphenyl)ethenyl]-1,3-benzenediol, cis-5-[2-(4-Hydroxyphenyl)ethenyl]benzene-1,3-diol, 05F9DB2A-D7E6-4063-8E5B-F7842CF74A5E, 5-[(1Z)-2-(4-Hydroxyphenyl)ethenyl]-1,3-benzenediol, InChI=1/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1-