ID: 4
InChIKey: UQRORFVVSGFNRO-UTINFBMNSA-N SMILES: CCCCN1C[C@@H]([C@H]([C@@H]([C@H]1CO)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function likely enhances CFTR function
Order of interaction unknown
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
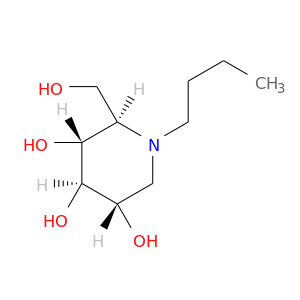
CID is 51634
synonyms found at PubChem are:
Miglustat, Zavesca, N-Butyldeoxynojirimycin, 72599-27-0, NB-DNJ, N-Butylmoranoline, N-butyl-1-deoxynojirimycin, BuDNJ, n-Butyl deoxynojirimycin, Butyldeoxynojirimycin, (2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol, n-Butyl dnj, N-(n-Butyl)deoxynojirimycin, SC-48334, Miglustat [USAN], OGT 918, N-Butyl-DNJ, SC 48334, miglustatum, Vevesca, UNII-ADN3S497AZ, Zavesca (TN), OGT-918, 3,4,5-Piperidinetriol, 1-butyl-2-(hydroxymethyl)-, (2R,3r,4R,5S)-, N-Bu-DNJ, Miglustat, Hydrochloride, N-Butyl-deoxynojirimycin, SC48334, ADN3S497AZ, CHEMBL1029, CHEBI:50381, D-Glucitol, 1,5-(butylimino)-1,5-dideoxy-, 1,5-Dideoxy-1,5-N-butylimino-D-glucitol, DSSTox_CID_25618, DSSTox_RID_81006, DSSTox_GSID_45618, N-(n-butyl)-1,5-dideoxy-1,5-imino-D-glucitol, 3,4,5-Piperidinetriol, 1-butyl-2-(hydroxymethyl)-, (2R-(2alpha,3beta,4alpha,5beta))-, NBV, CAS-72599-27-0, Brazaves, Miglustat [USAN:INN:BAN], NCGC00018140-02, Brazaves (TN), Miglustat (USAN/INN), AC1L1BHJ, D0HR8Z, Miglustat (JAN/USAN/INN), SCHEMBL246893, BICL4040, GTPL4841, DTXSID6045618, BDBM18355, UQRORFVVSGFNRO-UTINFBMNSA-N, HMS2090N20, ZINC3794711, Tox21_110830, 3917AH, AN-010, MFCD00272581, AKOS028109118, Tox21_110830_1, ACN-025888, API0003392, CS-2776, DB00419, NCGC00024452-03, NCGC00024452-04, BC677641, HY-17020, LS-116261, N-(n-Butyl)-1-deoxynojirimycin min. 99%, N-Butyldeoxynojirimycin, film (dried in situ), D05032, AB00489939-10, SR-01000000043, SR-01000000043-2, W-203639, 134282-77-2
ID: 789
InChIKey: HADVYIYJLZSYGH-BZNPZCIMSA-N SMILES: N(CC=C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
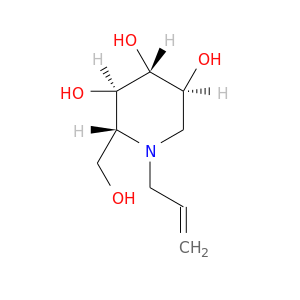
CID is 56952612
synonyms found at PubChem are:
SCHEMBL366469
ID: 3035
InChIKey: VQWYENSHOSDORF-UTINFBMNSA-N SMILES: N(CCC#C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
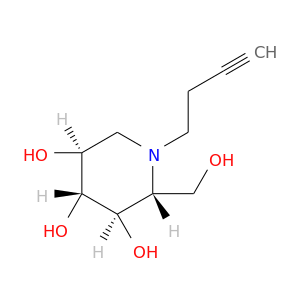
ID: 1197
InChIKey: KRNOSIJCJVCXKU-WRWGMCAJSA-N SMILES: CCCCCCN1C[C@@H]([C@H]([C@@H]([C@H]1CO)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
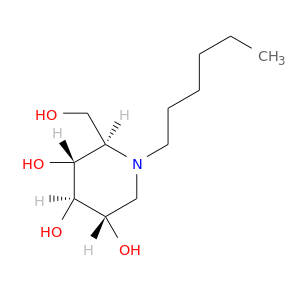
CID is 12766473
synonyms found at PubChem are:
N-Hexyl-DNJ, SCHEMBL2789051, CHEMBL1651550, BDBM18356, (2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-3,4,5-triol, (2R)-1-Hexyl-2alpha-(hydroxymethyl)piperidine-3beta,4alpha,5beta-triol
ID: 3064
InChIKey: YQPMMCSWGHFWEY-UTINFBMNSA-N SMILES: N(CCC=C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
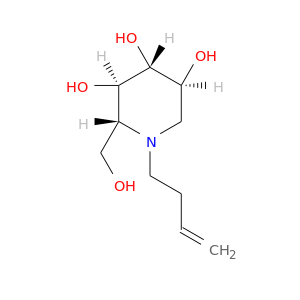
ID: 2888
InChIKey: BXIUMZGBYAQASY-HFYYSOHNSA-N SMILES: N(CC(C)F)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: Non-effective CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
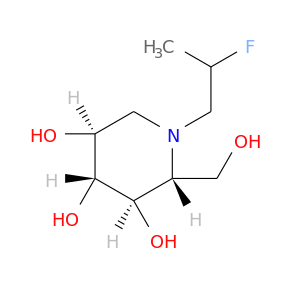
ID: 3072
InChIKey: ZOZLJFXFSKTQIQ-BZNPZCIMSA-N SMILES: N(CCC(F)(F)C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: Non-effective CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
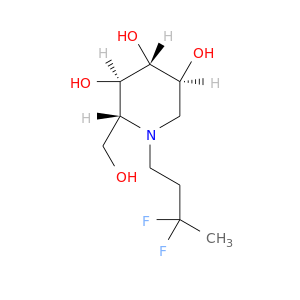
ID: 2998
InChIKey: PVGXJMXLVGVBKO-JNSGDMPLSA-N SMILES: N(CCC(F)C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: Non-effective CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
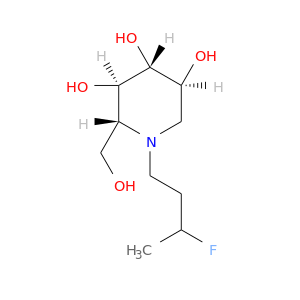
ID: 3032
InChIKey: VJJQODKBSDDABN-ULAWRXDQSA-N SMILES: N(CC(F)(F)C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: Non-effective CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
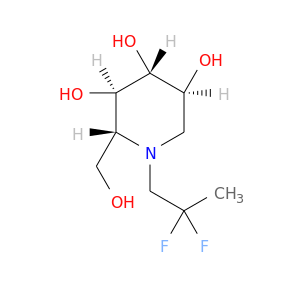
ID: 2645
InChIKey: XVZBPRMCKGPWFX-BZNPZCIMSA-N SMILES: N(CC#C)1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)C1
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
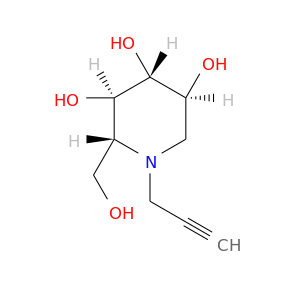
CID is 101518114
synonyms found at PubChem are:
1,5-Dideoxy-1,5-(propargylimino)-D-glucitol
ID: 2183
InChIKey: TYXAKMAAZJJHCB-XMTFNYHQSA-N SMILES: CCCCCCCCCCCCN1C[C@@H]([C@H]([C@@H]([C@H]1CO)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
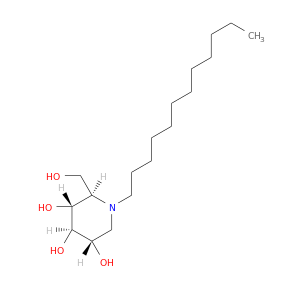
CID is 9818954
synonyms found at PubChem are:
N-dodecyldeoxynojirimycin, N-DODECYL-1-DEOXYNOJIRIMYCIN, CHEMBL450985, 79206-22-7, DTXSID20431312, BDBM50242628, ZINC44351037, N-Dodecyl-1-deoxynojirimycin, >=98.0% (TLC), W-203810
ID: 643
InChIKey: FTSCEGKYKXESFF-LXTVHRRPSA-N SMILES: CCCCCCCCCN1C[C@@H]([C@H]([C@@H]([C@H]1CO)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
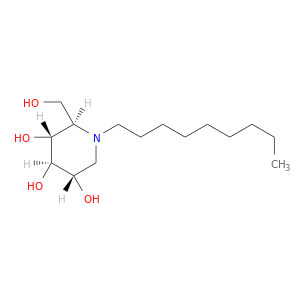
CID is 501640
synonyms found at PubChem are:
N-Nonyldeoxynojirimycin, N-(N-NONYL)DEOXYNOJIRIMYCIN, NN-DNJ, 81117-35-3, N-Nonyl-deoxynojirimycin, (2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-3,4,5-triol, CHEBI:76399, N-nonyl-1-deoxynojirimycin, N-Nonyl-DNJ, AC1L9V9L, N-Nonyl 1-Deoxynojirimycin, N-(n-Nonyl)-deoxynojirimycin, (2R,3R,4R,5S)-2-(Hydroxymethyl)-1-nonyl-3,4,5-piperidinetriol, BICL4182, CHEMBL408500, SCHEMBL2268575, BDBM18358, CTK8G1326, DTXSID60333407, MolPort-023-276-901, ZINC14253608, NN-DNJ, >=98% (HPLC), AKOS024457751, DB08283, NCGC00182087-01, N-(n-Nonyl)-1-deoxynojirimycin min. 99%, B7472, W-203849, (2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonyl-piperidine-3,4,5-triol, (5S,2R,3R,4R)-2-(Hydroxymethyl)-1-nonylpiperidine-3,4,5-triol, 3,4,5-Piperidinetriol, 2-(hydroxymethyl)-1-nonyl-, (2R,3R,4R,5S)-