ID: 12
InChIKey: WMBWREPUVVBILR-WIYYLYMNSA-N SMILES: C1[C@H]([C@H](OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O
biological descriptors:
CFTR relevance: CFTR activatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment several
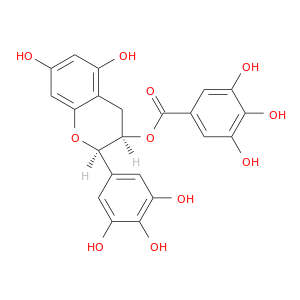
CID is 65064
synonyms found at PubChem are:
(-)-Epigallocatechin gallate, EGCG, Epigallocatechin gallate, 989-51-5, Epigallocatechin 3-gallate, Epigallocatechin-3-gallate, Tea catechin, (-)-Epigallocatechin-3-o-gallate, Teavigo, (-)-Epigallocatechol gallate, Epigallocatechin-3-monogallate, (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl 3,4,5-trihydroxybenzoate, Catechin deriv., UNII-BQM438CTEL, Green tea extract, CCRIS 3729, (-)-epigallocatechin 3-gallate, C22H18O11, BQM438CTEL, CHEBI:4806, CHEMBL297453, (2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl 3,4,5-trihydroxybenzoate, WMBWREPUVVBILR-WIYYLYMNSA-N, Epigallocatechol, 3-gallate, (-)-, (-)-Epigallocatechin gallate (EGCG), [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate, DSSTox_CID_567, DSSTox_RID_78830, DSSTox_GSID_29889, Sunphenon, [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 3,4,5-trihydroxybenzoate, EGCG cpd, (-)-Epigallocatechin gallate (85% (-)-epigallocatechin gallate, 10% (-)-epigallocatechin, 5% (-)- epicatechin gallate), (2R,3R)-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1[2H]-benzopyran-3,5,7-triol-3-(3,4,5-trihydroxybenzoate), CAS-989-51-5, SMR000449288, epigallo-catechin gallate, SR-01000759328, L-Epigallocatechin gallate, PF-EGCg 90, Epigallocate, NVP-XAA 723, (-)-EGCG, Epigallocic acid, Teavigo;, EGCG analogs, GTPs,Anagen, 2kdh, 3oob, 4awm, EGCG, Anagen, KDH, Epigallocatcchin Gallate, Spectrum_000316, SpecPlus_000277, Spectrum2_000168, Spectrum3_000244, Spectrum4_001541, Spectrum5_000102, Galloyl-L-epigallocatechol, D0E9DB, D0U2BH, AC1L22IG, AC1Q5X0Z, (-)-Epigallocatechin gallat, (-)-Epigallocatehin gallate, SCHEMBL35258, BSPBio_001628, KBioGR_002002, KBioSS_000796, SPECTRUM210239, cid_65064, EPIGALOCATECHIN GALLATE, MLS000758300, MLS001424000, DivK1c_006373, Green tea polyphenols, Anagen, SPBio_000035, Epigallocatechin gallate(EGCG), Epigallocatechin monogallate, B, GTPL7002, MEGxp0_001166, Epigallocatechin gallate, Anagen, DTXSID1029889, ACon1_001054, KBio1_001317, KBio2_000796, KBio2_003364, KBio2_005932, KBio3_001128, AOB2839, (-)-cis-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate, EGCG solution, 20 mM in DMSO, MolPort-001-741-358, (-)-EpigallocatechinGallateHydrate, HMS2051K21, HMS3649E08, ZINC3870412, Tox21_201468, Tox21_303457, BDBM50070942, CCG-38378, FR-109, LMPK12030005, MFCD00075940, s2250, AKOS015918182, ACN-035233, CS-1258, DB12116, DS-9030, EBD2138593, LS-2197, MCULE-3341525983, MCULE-7760530136, NC00078, SDCCGMLS-0066550.P001, (-)-Epigallocatechin gallate, >=95%, Epigallocatechin gallate analogs, Anagen, KS-0000132O, NCGC00164319-01, NCGC00164319-02, NCGC00164319-03, NCGC00164319-04, NCGC00164319-06, NCGC00257243-01, NCGC00259019-01, AN-15867, BP-30205, CPD000449288, HY-13653, K791, SAM001247031, SC-47284, ST097428, AB0014381, KB-206444, E0694, FT-0082796, FT-0604392, N1874, C09731, W-5069, (-)-Epigallocatechin gallate, >=97.0% (HPLC), (-)-Epigallocatechin gallate, analytical standard, 989E515, Epigallocatechin Gallate solution, 20 mM in DMSO, Gallic acid, 3-ester with epigallocatechol, (-)-, SR-01000946601, epigallocatechin-3-gallate solution, 20 mM in DMSO, I14-7747, Q-100914, SR-01000759328-5, SR-01000759328-6, SR-01000946601-1, (-)-EPIGALLOCATECHIN GALLATE (GREEN TEA EXTRACT), (-)-Epigallocatechin Gallate solution, 20 mM in DMSO, (-)-cis-3,3',4',5,5',7-Hexahydroxy-flavane-3-gallate, ANTIOXIDANT MODEL (TRAMP) -EPIGALLOCATECHIN GALLATE, UNII-T432289GYZ component WMBWREPUVVBILR-WIYYLYMNSA-N, (-)-Epigallocatechin gallate, >=80% (HPLC), from green tea, (-)-Epigallocatechin Gallate, disposable screening library format, Epigallocatechin gallate, primary pharmaceutical reference standard, (-)-Epigallocatechin-3-O-gallate, United States Pharmacopeia (USP) Reference Standard, (2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl 3,4,5-trihydroxyb enzoate, [5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 2,3,4-trihydroxybenzoate, Epigallocatechin gallate, Pharmaceutical Secondary Standard; Certified Reference Material, (-)-cis-3,4-Dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-1(2H)-benzopyran-3-yl Gallate, (2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate, (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl-3,4,5-trihydroxybenzoate, 3,4,5-Trihydroxybenzoic acid (2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester, 3,4-Dihydro-5,7-dihydroxy-2R-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3R-yl-3,4,5-trihydroxybenzoate, 863-65-0, Benzoic acid, 3,4,5-trihydroxy-, (2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester, Benzoic acid, 3,4,5-trihydroxy-, 3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester, (2R-cis)-, Benzoic acid, 3,4,5-trihydroxy-,(2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester
ID: 2817
InChIKey: ZJONSYFOVDKINV-UHFFFAOYSA-N SMILES: C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=C(C(=C(C=C4)O)O)O
biological descriptors:
CFTR relevance: proteostasis regulator (targeting disabled autophagy and CK2 overactivation)Category:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment several
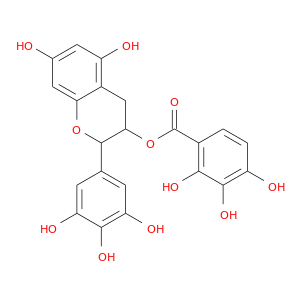
CID is 44134699
synonyms found at PubChem are:
(-) EPIGALLOCATECHIN GALLATE, BDBM84980, AC-390, AKOS015963174, BC215524, A845931, [5,7-bis(oxidanyl)-2-[3,4,5-tris(oxidanyl)phenyl]-3,4-dihydro-2H-chromen-3-yl] 2,3,4-tris(oxidanyl)benzoate, 2,3,4-trihydroxybenzoic acid [5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl] ester, 5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 2,3,4-trihydroxybenzoate
ID: 73
InChIKey: UFULAYFCSOUIOV-UHFFFAOYSA-N SMILES: C(CS)N
biological descriptors:
CFTR relevance: proteostasis regulator (targeting disabled autophagy and CK2 overactivation)Category:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment several
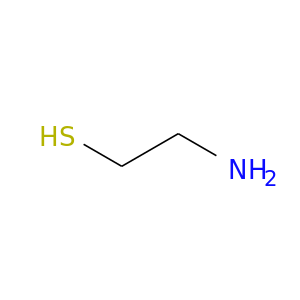
CID is 6058
synonyms found at PubChem are:
CYSTEAMINE, 2-Aminoethanethiol, Mercaptamine, 60-23-1, Thioethanolamine, Becaptan, Mercamine, Cysteinamine, beta-Mercaptoethylamine, 2-Mercaptoethylamine, Cysteamin, Lambraten, Lambratene, beta-Aminoethanethiol, Cystagon, Riacon, Decarboxycysteine, Mercaptoethylamine, 2-Amino-1-ethanethiol, Cisteamina, 2-Aminoethyl mercaptan, beta-Aminoethylthiol, 2-Mercaptoethanamine, Ethanethiol, 2-amino-, Mercaptamin, (2-Mercaptoethyl)amine, MEA (mercaptan), Ethanethiolamine, beta-MEA, Aminoethyl mercaptan, Mercaptamina, Mercaptaminum, Cysteamide, Mecramine, Mercamin, Merkamin, Cisteamina [Italian], Cystaran, 2-AMINO-ETHANETHIOL, Mercaptaminum [INN-Latin], Mercaptamina [INN-Spanish], 1-Amino-2-mercaptoethylamine, 2-aminoethane-1-thiol, Cysteamine [USAN:BAN], L-1573, Cysteamine (USAN), WR 347, Cystavision, C2H7NS, UNII-5UX2SD1KE2, Cysteamine bitartate, NSC 647528, Cysteamine [USAN], Mercaptamine (INN), CCRIS 3083, HSDB 7353, .beta.-Mercaptoethylamine, EINECS 200-463-0, L 1573, 5UX2SD1KE2, CHEBI:17141, UFULAYFCSOUIOV-UHFFFAOYSA-N, cysteaminium, 2-Mercaptoethylamine, polymer-bound, Mercaptamine [INN], NSC647528, NCGC00015691-03, (Mercaptoethyl)ammonium toluene-p-sulphonate, C-9500, 60-23-1 (Parent), Cystagone, b-Aminoethylthiol, 2-aminoethanethio, b-Aminoethanethiol, mercapto ethylamine, b-Mercaptoethylamine, DHL, 2-amino-ethyl thiol, CASH, .beta.-MEA, Cysteamine, ~95%, Cysteamine, free base, .beta.-Aminoethylthiol, Spectrum_001755, .beta.-Aminoethanethiol, ACMC-1AVBC, SpecPlus_000654, AC1L1LPL, Lopac-M-6500, DSSTox_CID_2875, bmse000388, CHEMBL602, D0V0LB, AC1Q54NL, DSSTox_RID_76770, DSSTox_GSID_22875, KBioSS_002235, KSC490G2L, DivK1c_006750, 156-57-0 (hydrochloride), 641022_ALDRICH, BDBM7968, GTPL7440, 3037-04-5 (tosylate), DTXSID3022875, CTK3J0325, KBio1_001694, KBio2_002235, KBio2_004803, KBio2_007371, KS-00000WES, 42954-15-4 (hydrobromide), Cysteamine, >=98.0% (RT), MolPort-001-662-635, BCP15015, ZINC8034121, EINECS 221-235-7, Tox21_113092, 16904-32-8 (di-hydrochloride), ANW-33452, HY-77591A, STK315355, AKOS003793343, CCG-204834, CS-2799, DB00847, MCULE-1838427828, NE18622, NSC-647528, RP18367, RTR-031658, CAS-60-23-1, NCGC00015691-01, NCGC00015691-02, NCGC00015691-04, NCGC00162236-01, NCGC00162236-02, AN-41805, BP-13401, KB-20296, LS-65761, NCI60_002000, SC-18413, SBI-0050727.P003, 27761-19-9 (tartrate (1:1)), 93965-19-6 (maleate (1:1)), AB0020070, DB-053562, TR-031658, A0648, FT-0611243, V0810, C01678, D03634, AB00053754_09, AB00053754_10, 106791-EP2292597A1, 106791-EP2295407A1, 106791-EP2298736A1, 213515-EP2371809A1, F0001-1576, 2DFDA1F8-7010-4225-8280-AB1C4C43F546, 139720-70-0, 2-Mercaptoethylamine, polymer-bound, 70-90 mesh, extent of labeling: 0.6-1.1 mmol/g loading, 1 % cross-linked with divinylbenzene, 2-ammonioethanethiolate, STL455134