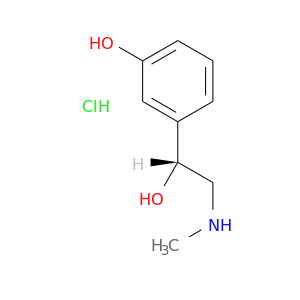
CID is 441279
synonyms found at PubChem are:
AC1L9AVO, [(2R)-2-hydroxy-2-(3-hydroxyphenyl)ethyl]-methylazanium chloride, Mydfrin, CHEBI:8094, AC1LCW6U, hydron; 3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol; chloride, PHENYLEPHRINE HYDROCHLORIDE, 61-76-7, Phenylephrine HCl, L-PHENYLEPHRINE HYDROCHLORIDE, (R)-Phenylephrine hydrochloride, Metaoxedrine chloride, Neosympatol, Synethenate, Almefrin, Consdrin, Emagrin, Fenilfar, Histabid, Idrianol, Lexatol, Oftalfrine, Phenistan, Pyristan, Stanephrin, Sucraphen, Synasal, Fenox, Alcon-efrin, meta-Sympatol, OP-Isophrin, Phenylephrine.HCl, Neo-Synesin 1, Neooxedrine chloride, Adrianol, Biomydrin, Neophryn, Prefrin, Rhinall, Consdrin hydrochloride, Isophrin hydrochloride, Vasosulf, Relief, Metaoxedrine hydrochloride, Neosynephrine hydrochloride, UNII-04JA59TNSJ, Op-Isophrin-Z, meta-Synephrine hydrochloride, Efricel, CCRIS 219, Levophenylephrine hydrochloride, Phenylephrine hydrochloride, l-, (-)-Phenylephrine hydrochloride, NCI-C55641, R-(-)-m-Synephrine hydrochloride, D-(-)-Phenylephrine hydrochloride, (R)-(-)-Phenylephrine hydrochloride, EINECS 200-517-3, m-Methylaminoethanolphenol hydrochloride, (R)-(-)-Phenylephrine (hydrochloride), 04JA59TNSJ, MLS000069696, Benzedrex Nasal Spray, Regular, (R)-3-(1-Hydroxy-2-(methylamino)ethyl)phenol hydrochloride, l-1-(m-Hydroxyphenyl)-2-methyl-aminoethanol hydrochloride, NCGC00024257-04, 3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol hydrochloride, SMR000058497, 1-m-Hydroxy-alpha-(methylaminomethyl)benzyl alcohol hydrochloride, (-)-m-Hydroxy-alpha-((methylamino)methyl)benzyl alcohol hydrochloride, (R)-3-Hydroxy-alpha-((methylamino)methyl)benzenemethanol hydrochloride, DSSTox_CID_1142, (-)-alpha-Hydroxy-beta-(methylamino)ethyl-alpha-(3-hydroxybenzene) hydrochloride, Benzenemethanol, 3-hydroxy-alpha-((methylamino)methyl)-, hydrochloride, (-)-, C9H13NO2.HCl, DSSTox_RID_75972, DSSTox_GSID_21142, Q-201561, Vazculep, (R)-(-)-1-(3-Hydroxyphenyl)-2-methylaminoethanol hydrochloride, Benzenemethanol, 3-hydroxy-alpha-((methylamino)methyl)-, hydrochloride (R)-, Benzyl alcohol, m-hydroxy-alpha-((methylamino)methyl)-, hydrochloride, (-)-, Sudafed PE, Benzenemethanol, 3-hydroxy-alpha-((methylamino)methyl)-, hydrochloride, (alphaR)-, CAS-61-76-7, L(-)-Phenylephrine hydrochloride, Incostop, 3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol;hydrochloride, SR-01000075286, Phenylephrine hydrochloride [USP:JAN], Vicks Sinex Vapospray, Neo-Synephrine (TN), Phenylephrine hydrochloride [USAN:JAN], PubChem23988, Vicks Sinex Nasal Spray, AC1NR4GJ, Opera_ID_1786, neosynephrine hydrocholride, Afrin 4 Hour Nasal Spray, D0D7QO, EC 200-517-3, SCHEMBL24654, MLS006012005, 59-42-7 (Parent), SPECTRUM1500483, L-Pheny lephrine Hydrochloride, Phenylephrine hydrochloride gel, Phenylephrine hydrochloride,(S), CHEMBL1200339, DTXSID3021142, VEN-308, MolPort-003-666-260, OCYSGIYOVXAGKQ-FVGYRXGTSA-N, HMS1920H08, Pharmakon1600-01500483, BCP23410, HY-B0471, Tox21_110897, Tox21_201261, Tox21_302846, Tox21_500920, CCG-39113, GL9617, MFCD00012605, NSC757273, s2569, AKOS022190146, Tox21_110897_1, CS-2585, LP00920, LS-1454, NSC-757273, Phenylephrine hydrochloride (JP17/USP), NCGC00024257-08, NCGC00094231-01, NCGC00256496-01, NCGC00258813-01, NCGC00261605-01, BCP0726000237, AB2000665, (R)-(-)-Phenylephrine hydrochloride, powder, EU-0100920, P0398, ST24035423, VU0239753-6, D00511, P 6126, 10% phenylephrine HCl cream, Solvay/SLA Pharma, 20% phenylephrine HCl cream, Solvay/SLA Pharma, Phenylephrine hydrochloride gel (fecal incontinence), SR-01000075286-1, (R)-(-)-Phenylephrine hydrochloride, analytical standard, UNII-O2VT86KV7E component OCYSGIYOVXAGKQ-FVGYRXGTSA-N, 3-Hydroxy-alpha-(methylaminomethyl)benzyl alcohol hydrochloride, (R)-(-)-Phenylephrine hydrochloride, tested according to Ph.Eur., (R)-3-hydroxy-a-[(methylamino)methyl]-benzenemethanol hydrochloride, (-)-3-hydroxy-alpha-((methylamino)methyl)benzenemethanol, hydroc hloride, (-)-3-hydroxy-alpha-((methylamino)methyl)benzenemethanol, hydrochloride, (R)-(-)-3-Hydroxy-alpha-(methylaminomethyl)benzyl alcohol hydrochloride, Benzenemethanol, 3-hydroxy-a-[(methylamino)methyl]-, hydrochloride,(aR)-, Phenylephrine hydrochloride, European Pharmacopoeia (EP) Reference Standard, Benzenemethanol, 3-hydroxy-alpha-((methylamino)methyl)-, hydrochloride (1:1), (alphaR)-, Phenylephrine hydrochloride gel (fecal incontinence), SLA Pharma/Ventrus Biosciences, Phenylephrine Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material, Phenylephrine hydrochloride, United States Pharmacopeia (USP) Reference Standard, 50741-76-9, 644-22-4, 827-62-3, Phenylephrine hydrochloride for peak identification, European Pharmacopoeia (EP) Reference Standard