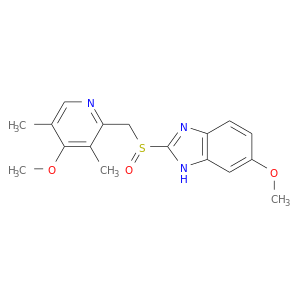
CID is 4594
synonyms found at PubChem are:
omeprazole, 73590-58-6, Losec, Prilosec, Antra, Esomeprazole, Omeprazon, Audazol, Omapren, Omepral, Parizac, Zegerid, Mopral, Miol, Belmazol, Ceprandal, Dizprazol, Dudencer, Emeproton, Epirazole, Gastrimut, Gastroloc, Gibancer, Indurgan, Inhibitron, Inhipump, Logastric, Pepticum, Peptilcer, Prazidec, Sanamidol, Secrepina, Ulcometion, Mepral, Miracid, Omeprol, Omezol, Omisec, Omizac, Ompanyt, Ozoken, Prysma, Ramezol, Ulceral, Ulcesep, Ulcozol, Zefxon, Zoltum, Desec, Elgam, Lomac, Ulsen, Ultop, Zimor, Ocid, Omed, Omid, OMEP, Demeprazol, Nopramin, Omeprazol, Omezolan, Paprazol, Pepticus, Prazentol, Prazolit, Procelac, Regulacid, Danlox, Erbolin, Lensor, Morecon, Nilsec, Olexin, Omegast, Omesek, Ortanol, Osiren, Proclor, Result, Ulcsep, Victrix, Zepral, Exter, Gasec, Ulzol, Omebeta 20, Tedec Ulceral, AULCER, Antra MUPS, Omeprazolum, Omez, Prilosec OTC, Omepradex, Omerprazole, Nexium IV, H 168/68, Omeprazol [INN-Spanish], Omeprazolum [INN-Latin], Esomperazole, Gastrogard, Nuclosina, Emilok, r-omeprazole, Omeprazole magnesium, ( -)-Omeprazole, Omeprazone, Omesec, Omeprazole delayed-release, Prilosec (TN), Prestwick_808, OMEPRAZOLE SODIUM, CCRIS 7099, OMZ, CHEBI:77260, HSDB 3575, Esomeprazole sodium salt, Omeprazole [USAN:INN:BAN:JAN], 5-Methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole, Nexium, H-168/68, CHEMBL1503, Nexiam, 1H-Benzimidazole, 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-, 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1H-1,3-benzodiazole, 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole, MLS000069373, SAN-15, C17H19N3O3S, AGI-010, SUBDBMMJDZJVOS-UHFFFAOYSA-N, (+)-omeprazole, H 168-68, 2-(((3,5-Dimethyl-4-methoxy-2-pyridyl)methyl)sulfinyl)-5-methoxy-1H-benzimidazole, DM-3458, NCGC00016925-06, SMR000058847, CAS-73590-58-6, Esomeprazole Sodium, O0359, DSSTox_CID_1080, 5-Methoxy-2[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]-1H-benzimidazole, 2-({[3,5-dimethyl-4-(methyloxy)pyridin-2-yl]methyl}sulfinyl)-5-(methyloxy)-1H-benzimidazole, 6-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole, DSSTox_RID_75929, UNII-S51HU491WJ, DSSTox_GSID_21080, Omebeta, Olit, Omeprazen, 5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl)-1H-benzo[d]imidazole, 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]-1H-benzimidazole, 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1H-benzimidazole, (R)-Esomeprazole, (+-)-Omeprazole, Omeprazole, (R)-, Omeprazole (JAN/USP/INN), SR-01000003003, R-Omeprazole [USP-RS], S51HU491WJ, Omperazole, 161796-78-7, Omeprazole, solid, 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1H-benzimidazole, 6-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]-1H-benzimidazole, Omeprazole Pellets, Omeprazole,(S), Losec (TN), Losec, Omesec, Prilosec, Zegerid, Omeprazole, Omeprazole [USAN:USP:INN:BAN:JAN], (s)-omeprazole sodium, Omeprazole (Prilosec), ACMC-20p1du, AC1L1IIJ, Maybridge4_002645, Opera_ID_1863, Prestwick0_000493, Prestwick1_000493, Prestwick2_000493, Prestwick3_000493, (.+/-.)-Omeprazole, Omeprazole, 98% 1g, D01XNB, UPCMLD-DP075, cid_4594, SCHEMBL1191, omeprazole sodium bicarbonate, BSPBio_000385, MLS001076112, MLS001424148, MLS006010400, MLS006011759, BIDD:GT0189, SPBio_002306, BPBio1_000425, GTPL4279, Omeprazole (JP17/USP/INN), Esomeprazole sodium salt hydrate, DTXSID6021080, SCHEMBL11995456, UPCMLD-DP075:001, CHEBI:91766, CTK8I2124, CTK9A5793, MolPort-003-666-741, MolPort-003-849-702, HMS1528I05, HMS1569D07, HMS2052G17, HMS2090E16, HMS2090F11, HMS2096D07, HMS2232B21, HMS3269D17, HMS3394G17, HMS3651A11, HMS3713D07, Pharmakon1600-01505693, (S)-Omeprazole sodium salt hydrate, BCP05852, BCP13592, BCP21299, HY-B0113, KS-000001KH, 2,3,5-Trimethylpyridine/Omeprazole, Tox21_110686, Tox21_200509, AC-401, BBL028172, BDBM50103597, BDBM50241343, CO0037, DL-462, MFCD00083192, NSC751450, NSC759192, s1389, STK623746, 119141-88-7 (base), AKOS005066653, AKOS015895343, Tox21_110686_1, AC-4676, CCG-101130, CCG-213517, CS-1868, DB00338, HS-0055, LS-7629, MCULE-3224208952, NC00380, NE55490, NSC-751450, NSC-759192, 1H-Benzimidazole, 6-methoxy-2-((R)-((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-, IDI1_032523, NCGC00016925-01, NCGC00016925-02, NCGC00016925-03, NCGC00016925-04, NCGC00016925-05, NCGC00016925-07, NCGC00016925-08, NCGC00016925-10, NCGC00016925-11, NCGC00021522-03, NCGC00021522-04, NCGC00021522-05, NCGC00258063-01, Omeprazole, analytical reference material, AN-11759, AN-15878, BC203197, BC226373, CPD000058847, K253, KB-63582, SAM001246900, SC-15162, SBI-0206896.P001, AB0014122, AB1009257, TL8005099, FT-0601585, FT-0652860, FT-0653294, H 199, ST24048844, A19447, C07324, D00455, J10125, 668985-31-7 (Mg), 141O887, A837865, H168/68, I06-0705, SR-01000003003-4, SR-01000003003-7, SR-01000003003-8, 5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl), BRD-A55962179-001-04-9, BRD-A55962179-001-08-0, BRD-A55962179-001-20-5, BRD-A88691025-001-07-4, I14-40975, F0001-2386, Z1672902589, Omeprazole, European Pharmacopoeia (EP) Reference Standard, Omeprazole, United States Pharmacopeia (USP) Reference Standard, 2-(3-methoxy-2,4-dimethylbenzylsulfinyl)-6-methoxy-1H-benzo[d]imidazole, 5-Methoxy-2-((S)-((4-methoxy-3,5-dimethyl-2- pyridinyl)methyl)sulfinyl)-, Omeprazole, Pharmaceutical Secondary Standard; Certified Reference Material, (+)-5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-sulfinyl]-1h-benzimidazole, (-)-5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-sulfinyl]-1h-benzimidazole, (RS)-5-Methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridylmethylsulphinyl)benzimidazole, 5-methoxy 2-[[(4-methoxy-3,5-dimethyl-2-pyrdinyl)-methyl]sulfinyl]-1H-benzimidazole, 5-methoxy 2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1 H-benzimidazole, 5-methoxy 2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1H-benzimidazole, 5-methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole, 5-methoxy-2-(2-(4-methoxy-3,5-dimethylpyridin-2-yl)ethylsulfinyl)-1H-benzo[d]imidazole, 5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole, 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl- 1H-benzimidazole, 5-methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]-3H-benzoimidazole, 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-3H-1,3-benzodiazole, 5-Methoxy-2-[(RS)-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-1H-benzimidazole, 5-methoxy-2-[[(3,5-dimethyl-4-methoxy-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulphinyl]-1H-benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-benzimidazole, 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfonyl]benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulphinyl]1H-benzimidazole, 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methane]sulfinyl}-1H-1,3-benzodiazole, 6-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1H-benzimidazole, 6-methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]-1H-benzimidazole, 6-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole, 6-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1h-benzimidazole, 6-methoxy-2-[[(4-methoxy-3,5dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole, 6-methoxy-2-[[(4-methoxy3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1h-benzimidazole, 6-methoxy-2-[[(4methoxy-3,5-dimethyl2-pyridinyl)methyl]sulfinyl]-1h-benzimidazole, 6-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methane]sulfinyl}-1H-1,3-benzodiazole, Omeprazole for peak identification, European Pharmacopoeia (EP) Reference Standard, Omeprazole solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, (omeprazole)5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole, (RS)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl) methylsulfinyl)-1H-benzo[d]imidazole, 131959-78-9, 172964-80-6, 177541-02-5, 5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole (omeprazole), 5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole(Omeprazole), 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole; Antra; Losec, 6-Methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole sodium salt