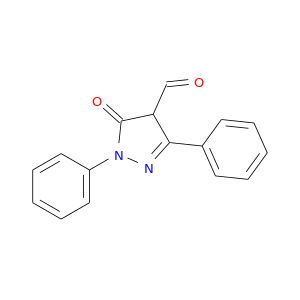
CID is 205105
synonyms found at PubChem are:
1,3-Diphenyl-4-formyl-2-pyrazolin-5-one, 17364-40-8, 2-Pyrazolin-5-one, 1,3-diphenyl-4-formyl-, 5-oxo-1,3-diphenyl-4H-pyrazole-4-carbaldehyde, AC1L4DYX, SCHEMBL17290527, LS-128870, 1,3-Diphenyl-5-oxo-2-pyrazoline-4-carbaldehyde, 5-oxo-1,3-diphenyl-4,5-dihydro-1H-pyrazole-4-carbaldehyde