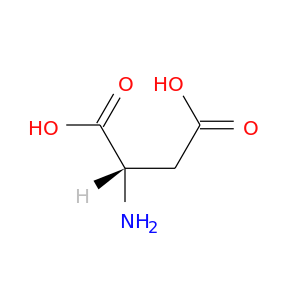
CID is 5960
synonyms found at PubChem are:
L-aspartic acid, aspartic acid, 56-84-8, H-Asp-OH, L-aspartate, Asparagic acid, Aspatofort, L-Asparagic acid, L-Aminosuccinic acid, (S)-2-Aminosuccinic acid, Asparaginic acid, L-Asparaginic acid, (2S)-2-aminobutanedioic acid, (S)-Aspartic acid, (2S)-Aspartic acid, aspartate, (S)-Aminobutanedioic acid, L-Aspartinsaeure, L-Asparaginsaeure, L-Asparaginsyra, Aspartic acid, L-, Acidum asparticum, L-(+)-Aspartic acid, L-Asp, L-2-Aminobutanedioic acid, Aspartate, L-, Asparaginsaeure [German], Aminosuccinic acid, Butanedioic acid, amino-, (S)-, Aspartic acid (VAN), Asparagic acid (VAN), Asparaginic acid (VAN), Acide aspartique [INN-French], Acido aspartico [INN-Spanish], (L)-ASPARTIC ACID, (+)-Aspartic acid, (S)-(+)-Aminosuccinic acid, FEMA No. 3565, CCRIS 6181, Acidum asparticum [INN-Latin], HSDB 1430, L( )-Aminobernsteinsaeure, AI3-04461, NSC 3973, Aspartic Acid [USAN:INN], BRN 1723530, l(+)-aspartic acid, Calcium diaspartate, EINECS 200-291-6, 25608-40-6, (S)-(+)-Aspartic acid, (S)-2-aminobutanedioic acid, CHEMBL274323, 6899-03-2, asp, CHEBI:17053, UNII-Z4FZM6CA61, CKLJMWTZIZZHCS-REOHCLBHSA-N, Calcium dihydrogen di-L-aspartate, (S)-Aspartate, (+)-Aspartate, [3H]-L-aspartate, L-Aspartic acid-13C4, 1-Amino-1,2-carboxyethane, (S)-Aminobutanedioate, [3H]L-aspartic acid, [3H]-L-aspartic acid, Asparaginsaeure, Polysuccinimide, (2S)-2-azanylbutanedioic acid, Acido aspartico, Z4FZM6CA61, Acide aspartique, 39162-75-9, 56-84-8 (Parent), Polyaspartic acid, Poly-DL-succinimide, 55443-54-4, 4-04-00-02998 (Beilstein Handbook Reference), L(+)-Aminobernsteinsaeure, L-Aspartic acid homopolymer, Aminosuccinate, Asparagate, Asparatate, L-Aspartic acid, homopolymer, L-Asparagate, L-Aminosuccinate, Aspartic acid [USAN:USP:INN], (L)-Aspartate, L- Aspartic acid, alpha-Aminosuccinate, (2S)-Aspartate, EINECS 254-327-0, L-(+)-Aspartate, L-[14C]aspartate, Beta-L-Aspartic Acid, L-Aspartic acid, 2, (R)-2-aminosuccinate, (S)-2-aminosuccinate, Tocris-0214, [3h]-l-asp, (S)-amino-Butanedioate, alpha-Aminosuccinic acid, (S)-Aminosuccinic Acid, (S)-(+)-Aspartate, L-Aspartic acid (9CI), 2-Amino-3-methylsuccinate, Biomol-NT_000168, bmse000031, bmse000875, D0OT9B, Aspartic acid (USP/INN), EC 200-291-6, AC1L1LI6, L-Aspartic acid (JP17), SCHEMBL3231, (S)-amino-Butanedioic acid, L-Aspartic acid, >=98%, L-Aspartic acid, 99.0%, Lopac0_000133, Aspartic acid, L- (8CI), KSC269K5J, DL-Aspartic acid, homopolymer, AC1Q4U77, BPBio1_001128, GTPL3309, GTPL4534, DTXSID7022621, aspartic acid (L-aspartic acid), BDBM18125, CTK1G9554, L-Aspartic acid, >=98%, FG, MolPort-001-792-017, HMS3260K08, ZINC895032, .alpha.-Aminosuccinic acid, (L)-, 21059-46-1 (calcium salt), 14007-45-5 (potassium salt), Tox21_500133, 2001-89-0 (di-potassium salt), ANW-32591, MFCD00002616, PDSP1_000819, PDSP2_000806, 1115-63-5 (mono-potassium salt), 17090-93-6 (hydrochloride salt), AKOS006239578, AKOS015853957, 5598-53-8 (di-hydrochloride salt), AM81585, CCG-204228, DB00128, LP00133, LS-2569, MCULE-6700641640, RP16381, RP20039, RTC-066533, 3792-50-5 (mono-hydrochloride salt), NCGC00024499-01, NCGC00024499-02, NCGC00024499-03, NCGC00024499-04, NCGC00024499-05, NCGC00024499-06, NCGC00260818-01, 27881-03-4, AJ-24135, AN-23565, BP-13291, KB-53137, SC-05405, SC-91380, TL806193, L-Aspartic acid, Vetec(TM) reagent grade, 2068-80-6 (magnesium (2:1) salt), AB1002629, DB-029944, KB-211185, ST2414603, TC-066533, 39162-75-9 (calcium (2:1) salt), A0546, EU-0100133, FT-0627708, FT-0655880, FT-0693446, L-Aspartic acid, BioXtra, >=99% (HPLC), V1940, L-Aspartic acid, BioUltra, >=99.5% (T), A 9256, C-4546, C00049, D00013, M03000, L-Aspartic acid, SAJ special grade, >=99.0%, A817928, A824434, L-Aspartic acid, reagent grade, >=98% (HPLC), SR-01000597734, I04-1073, I04-1122, I14-2732, SR-01000597734-3, (S)-(+)-Aminosuccinic acid; (S)-Aminobutanedioic acid, F8889-8684, Z1270403519, A4B5FB11-A4B6-4D75-9860-2ACF670700B9, UNII-28XF4669EP component CKLJMWTZIZZHCS-REOHCLBHSA-N, UNII-30KYC7MIAI component CKLJMWTZIZZHCS-REOHCLBHSA-N, Aspartic acid, European Pharmacopoeia (EP) Reference Standard, L-Aspartic acid, certified reference material, TraceCERT(R), Aspartic acid, United States Pharmacopeia (USP) Reference Standard, L-Aspartic acid, BioReagent, suitable for cell culture, suitable for insect cell culture, L-Aspartic acid, Pharmaceutical Secondary Standard; Certified Reference Material, 1236300-18-7, 155436-59-2, 155436-61-6, 155436-63-8, 155436-65-0, 181119-33-5, 181119-34-6, 221628-95-1, 26834-87-7, 90819-17-3, L-Aspartic acid, from non-animal source, meets EP, USP testing specifications, suitable for cell culture, 98.5-101.0%, L-Aspartic acid, PharmaGrade, Ajinomoto, EP, JP, USP, manufactured under appropriate GMP controls for Pharma or Biopharmaceutical production, suitable for cell culture, D09BKX