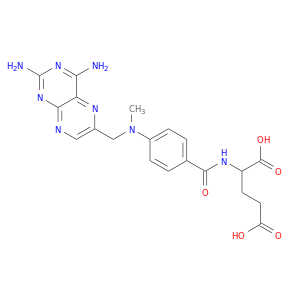
CID is 4112
synonyms found at PubChem are:
Amethopterin, DL-Amethopterin, (+)-Amethopterin, DL-Methotrexate, Methylaminopterin, 60388-53-6, TCMDC-123832, 6-Mtx (DL), Amethopterin (R,S), EINECS 262-213-7, METHOTREXATE(+/-), MLS002701970, N-(4-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)-DL-glutamic acid, N-(p-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)-DL-glutamic acid, NSC 117356, Glutamic acid, N-(p-(((2,4-diamino-6-pteridinyl)methyl)methylamino)benzoyl)-, DL-, L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-, Emtexate, Folex, C19H23N9O4, C20H22N8O5, 2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)formamido]pentanedioic acid, DL-Glutamic acid, N-(4-(((2,4-diamino-6-pteridinyl)methyl)methylamino)benzoyl)-, (+-)-Methotrexate, D(-)-AMETHOPTERIN, SR-05000001673, Methotrexate polyglutamate, FBOZXECLQNJBKD-UHFFFAOYSA-N, NSC117356, ( )Amethopterin, 2-[(4-{[(2,4-diaminopteridin-6-yl)methyl]methylamino}phenyl)carbonylamino]pent anedioic acid, Prestwick_753, Folex (Salt/Mix), AC1L1HFS, Prestwick0_000373, Prestwick1_000373, Prestwick2_000373, Prestwick3_000135, Prestwick3_000373, Methotrexate, (+/-)-, CHEMBL426, AC1Q5SF6, Methotrexate, (DL)-Isomer, SCHEMBL3712, BSPBio_000210, BSPBio_000525, BSPBio_001993, ChEMBL_59579, METHOTREXATE, U.S.P., SPECTRUM1500398, SPBio_002446, BPBio1_000232, BPBio1_000579, Methotrexate pound>>Amethopterin, SCHEMBL11986730, CHEBI:93775, Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)methylamino)benzoyl)-L-glutamova, MolPort-001-779-666, HMS1569K07, HMS1920L21, HMS2091D16, HMS2096K07, HMS3267F07, HMS3371G08, HMS3403O03, HMS3654M22, HMS3713K07, Pharmakon1600-01500398, BCP02078, BDBM50004545, METHOTREXATE USP AND EP GRADE, NSC757113, AKOS000281496, AKOS024282621, 4-Amino-N10-methylpteroylglutamic acid, CCG-212689, MCULE-3234195945, NSC-117356, NSC-757113, NCGC00015079-03, NCGC00015079-04, NCGC00095284-01, NCGC00095284-02, 4CA-0097, 82334-40-5, AC-11680, AN-39412, L-4-Amino-N10-methylpteroylglutamic acid, LS-71811, NCI60_041622, Poly(imino(1-carboxy-4-oxo-1,4-butanediyl)), alpha-(4-(((2,4-diamino-6-pteridinyl)methyl)methylamino)benzoyl)-omega-hydroxy-, (S)-, SMR001565548, ST057254, ST069344, SBI-0053664.P003, AB0012834, Kyselina 4-amino-N10-methylpteroylglutamova, M1664, 388A536, I06-0203, SR-05000001673-1, SR-05000001673-2, BRD-A55424491-001-07-4, BRD-A55424491-001-08-2, I14-19311, (MTX)2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid, 2-(4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)pentanedioic acid, 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioic acid, 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid, 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(MTX), Glutamic acid, N-[p-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-, L-(+)-, N-(4-[[(2,4-Diamino-6-pteridinyl)methyl](methyl)amino]benzoyl)glutamic acid, (L)-, N-(p-((2,4-Diamino-6-pteridyl)methyl)methylamino)benzoyl)glutamic acid, (L)-, (methotrexate)2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid, (Methotrexate, MTX)2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid, 184883-35-0, 2-[(4-{[(4-amino-2-imino-3H-pteridin-6-yl)methyl](methyl)amino}phenyl)formamido]pentanedioic acid, 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid (Methotrexate), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid (MTX), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid bis-adamantan-1-ylamide, 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(Amethopterin), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(Methotrexate (MTX)), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(Methotrexate ), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(methotrexate(MTX)), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(Methotrexate), 2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid(Methotrexate, MXT), 4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraene-10,14-diol(methotrexate, MTX), Methotrexate solution, 1.0 mg/mL in methanol with 0.1N NaOH, ampule of 1 mL, certified reference material, Methotrexate2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid, Poly(imino((1S)-1-carboxy-4-oxo-1,4-butanediyl)), alpha-(4-(((2,4-diamino-6-pteridinyl)methyl)methylamino)benzoyl)-omega-hydroxy-