"BR Grubb"
"A Mengos"
"RC Boucher"
"JR Riordan"
"ML Drumm"
"MR Knowles"
"SE Gabriel"
"AM Van Heeckeren"
"M Gentzsch"
"SH Randell"
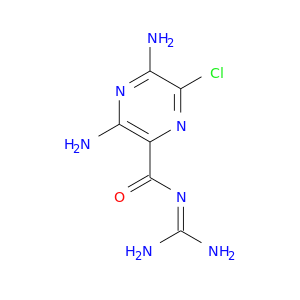
CID is 16231
synonyms found at PubChem are:
AMILORIDE, Amipramidin, Midamor, 2609-46-3, Guanamprazine, Amilorida, Amipramizid, Amipramizide, Guanamprazin, Amiloridum, Amyloride, Amiloridum [INN-Latin], Amilorida [INN-Spanish], Amiloride HCL, Amiloride [INN:BAN], N-Amidino-3,5-diamino-6-chloropyrazinecarboxamide, 3,5-diamino-N-carbamimidoyl-6-chloropyrazine-2-carboxamide, Amiprazidine, C6H8ClN7O, UNII-7DZO8EB0Z3, Amiclaran (TN), Amiloride (INN), CCRIS 6545, EINECS 220-024-7, N-Amidino-3,5-diamino-6-chlorpyrazincarboxamid, Amiloride hydrochloride hydrate, CHEMBL945, Pyrazinecarboxamide, 3,5-diamino-N-(aminoiminomethyl)-6-chloro-, 7DZO8EB0Z3, CHEBI:2639, 3,5-Diamino-N-(aminoiminomethyl)-6-chloropyrazinecarboxamide, Amikal (Hydrochloride dihydrate), Midamor (Hydrochloride dihydrate), XSDQTOBWRPYKKA-UHFFFAOYSA-N, 3,5-diamino-6-chloro-N-(diaminomethylidene)pyrazine-2-carboxamide, MK-870 (Hydrochloride dihydrate), N-amidino-3,5-diamino-6-chloro-2-pyrazinecarboxamide, NCGC00015089-08, AMIPRAMIDINE, DSSTox_CID_23853, DSSTox_RID_80077, Amiloride Hydrocholride, DSSTox_GSID_43853, AMILORIDE (SEE ALSO: AMILORIDE HCL (2016-88-8)), 3,5-diamino-N-[amino(imino)methyl]-6-chloropyrazine-2-carboxamide, Pyrazinecarboxamide, 3,5-diamino-N-(aminoiminomethyl)-6-chloro-, monohydrochloride, 137053-86-2, CAS-2609-46-3, Amiclaran, Amilorid, (3,5-diamino-6-chloropyrazin-2-yl)-N-(???methyl)carboxamide, Biduret (TN), Spectrum_000034, Tocris-0890, 1f5l, AC1Q3POC, Prestwick0_000007, Prestwick1_000007, Prestwick2_000007, Prestwick3_000007, Spectrum2_000118, Spectrum3_000293, Spectrum4_000132, Spectrum5_000776, Lopac-A-7410, D0I0RJ, AC1L27IZ, Lopac0_000111, SCHEMBL27562, BSPBio_000013, BSPBio_001572, BSPBio_001826, KBioGR_000292, KBioGR_000544, KBioSS_000292, KBioSS_000394, MLS001060798, BIDD:GT0466, DivK1c_000182, SPBio_000136, SPBio_001934, BPBio1_000015, GTPL2421, DTXSID9043853, BCBcMAP01_000101, BDBM16173, KBio1_000182, KBio2_000292, KBio2_000394, KBio2_002860, KBio2_002962, KBio2_005428, KBio2_005530, KBio3_000583, KBio3_000584, KBio3_001326, Amiloride (Na-Ca chanel blocker), MolPort-005-934-472, NINDS_000182, 17440-83-4 (hydrochloride), Bio1_000359, Bio1_000848, Bio1_001337, Bio2_000292, Bio2_000772, HMS1791O14, HMS1989O14, HMS2089H05, HMS2213E05, HMS3355K04, ACT05635, ACT05652, BCP16815, HY-B0285, ZINC4340269, Tox21_110080, BBL028157, SBB037856, STL373007, AKOS015961348, Tox21_110080_1, API0000380, CCG-204206, CS-2297, DB00594, LS-1094, MCULE-5948863568, 2016-88-8 (anhydrous hydrochloride), IDI1_000182, IDI1_034042, NCGC00015089-01, NCGC00015089-02, NCGC00015089-03, NCGC00015089-04, NCGC00015089-05, NCGC00015089-06, NCGC00015089-07, NCGC00015089-09, NCGC00015089-11, NCGC00015089-12, NCGC00015089-13, NCGC00015089-14, NCGC00015089-15, NCGC00015089-16, NCGC00015089-17, NCGC00024443-02, NCGC00024443-05, NCGC00024443-06, NCGC00024443-07, NCGC00024443-09, AC-13631, SMR000486264, ST079279, U460, (3,5-Diamino-6-chloropyrazinoyl)guanidine, SBI-0050099.P004, N-amidino-3,5-diamino-6-chloropyrazinamide, AB00053415, FT-0703177, C06821, D07447, EN300-149459, AB00053415-24, AB00053415-25, AB00053415_26, AB00053415_27, AB00053415_28, 117188-EP2277879A1, 117188-EP2298776A1, 609A463, J-016249, BRD-K97181089-003-02-3, BRD-K97181089-310-03-0, N-amidino 3,5-diamino-6-chloro-2-pyrazinecarboxamide, F2173-0531, 3,5-diamino-N-carbamimidoyl-6-chloro-pyrazine-2-carboxamide, 3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinamide;hydrochloride