ID: 1
InChIKey: TZBJGXHYKVUXJN-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment several
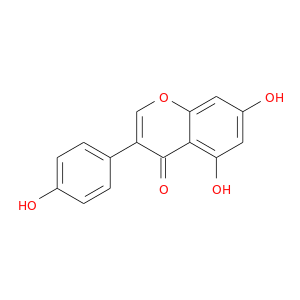
CID is 5280961
synonyms found at PubChem are:
genistein, 446-72-0, Prunetol, Genisteol, 4',5,7-Trihydroxyisoflavone, Genisterin, Sophoricol, 5,7,4'-Trihydroxyisoflavone, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one, Bonistein, Genestein, Differenol A, NPI 031L, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, C.I. 75610, SIPI 807-1, 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one, UNII-DH2M523P0H, NSC 36586, 4,5,7-Trihydroxyiso-flavone, Lactoferrin-genistein, CCRIS 7675, 4',5, 7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone, NSC36586, CHEMBL44, EINECS 207-174-9, IN1327, BRN 0263823, PTI G4660 (Genistein), ISOFLAVONE, 4',5,7-TRIHYDROXY-, MLS000738127, DH2M523P0H, STO514, CHEBI:28088, PTI-G4660, SIPI-9764-I, TZBJGXHYKVUXJN-UHFFFAOYSA-N, 4′,5,7-Trihydroxyisoflavone, TNP00151, NSC-36586, GENISTEIN (ENDOCRINE DISRUPTER), GEN, NCGC00015479-09, DSSTox_CID_2308, G 6649, G10000, DSSTox_RID_76542, DSSTox_GSID_22308, ENDOCRINE DISRUPTOR (GENISTEIN) (SEE ALSO GENISTEIN (446-72-0)), CAS-446-72-0, SR-01000075498, HSDB 7475, PTI G4660, 3kgt, 3kgu, 4&prime, Genistein, 8, Genistein,(S), PTI-G 4660, Genistein (flavonoid), PubChem9852, Spectrum_000320, Tocris-1110, 1x7r, 2qa8, Genistein 85% HPLC, SpecPlus_000305, AC1NQXT4, Spectrum2_000638, Spectrum3_000678, Spectrum4_001543, Spectrum5_000106, Lopac-G-6649, 4',7-Trihydroxyisoflavone, D0L4FS, MolMap_000022, UPCMLD-DP096, 4,5,7-Trihydroxyisoflavone, 4,6,7-Trihydroxyisoflavone, Isoflavone,5,7-trihydroxy-, Lopac0_000520, Oprea1_224620, Oprea1_437815, SCHEMBL19166, BSPBio_002375, KBioGR_002006, KBioGR_002564, KBioSS_000800, KBioSS_002573, SPECTRUM210296, 5-18-04-00594 (Beilstein Handbook Reference), BIDD:ER0113, DivK1c_006401, Genistein, analytical standard, SPBio_000636, 4',5,7-Trihydroxy isoflavone, 4',5,7-trihydroxy-Isoflavone, GTPL2826, MEGxp0_000568, 4,5,7-Trihydroxy Iso-Flavone, DTXSID5022308, UPCMLD-DP096:001, ACon1_001065, BDBM19459, cid_5280961, KBio1_001345, KBio2_000800, KBio2_002564, KBio2_003368, KBio2_005132, KBio2_005936, KBio2_007700, KBio3_001595, KBio3_003042, AOB5073, CHEBI: 28088, cMAP_000086, MolPort-000-003-911, Bio1_000445, Bio1_000934, Bio1_001423, HMS2271K09, HMS3261H21, HMS3267K14, HMS3428M01, HMS3649B22, HMS3654D17, ACT05962, ALBB-015886, BCP07581, KS-00000MH8, Prunetol solution, 20 mM in DMSO, Tox21_110161, Tox21_201428, Tox21_300585, Tox21_500520, AC-472, BBL010484, CCG-38551, CG-009, Genistein solution, 20 mM in DMSO, LMPK12050218, MFCD00016952, s1342, SBB066115, STK801619, ZINC18825330, AKOS001590147, Tox21_110161_1, CS-1534, DB01645, KS-5128, LP00520, LS-1266, MCULE-4857649752, RL03694, RP29616, SMP1_000133, Genistein; 4',5,7-Trihydroxyisoflavone, NCGC00015479-01, NCGC00015479-02, NCGC00015479-04, NCGC00015479-05, NCGC00015479-06, NCGC00015479-07, NCGC00015479-08, NCGC00015479-10, NCGC00015479-11, NCGC00015479-12, NCGC00015479-13, NCGC00015479-14, NCGC00015479-15, NCGC00015479-16, NCGC00015479-17, NCGC00015479-18, NCGC00015479-19, NCGC00015479-20, NCGC00025005-01, NCGC00025005-02, NCGC00025005-03, NCGC00025005-04, NCGC00025005-05, NCGC00025005-06, NCGC00025005-07, NCGC00169711-01, NCGC00169711-02, NCGC00254275-01, NCGC00258979-01, NCGC00261205-01, 690224-00-1, AJ-70669, AN-15821, BC200563, HY-14596, KB-52241, NCI60_003369, SC-04581, SMR000112580, ST056352, AB1004490, EU-0100520, FT-0603395, G0272, N1861, Genistein, disposable screening library format, 46G720, C06563, G-2535, Genistein, synthetic, >=98% (HPLC), powder, J10015, K00046, S-7751, US8552057, 2, AB00052696_09, AB00052696_12, A826657, Genistein, primary pharmaceutical reference standard, I06-0431, Q-100484, SR-01000075498-1, SR-01000075498-3, SR-01000075498-6, 3-(4-hydroxyphenyl)-5,7-bis(oxidanyl)chromen-4-one, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone, BRD-K43797669-001-02-3, BRD-K43797669-001-03-1, BRD-K43797669-001-10-6, Genistein, from Glycine max (soybean), ~98% (HPLC), SR-01000075498-10, 5,7-dihydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one, F0001-2388, 4H-1-Benzopyran-4-one,7-dihydroxy-3-(4-hydroxyphenyl)-, UNII-71B37NR06D component TZBJGXHYKVUXJN-UHFFFAOYSA-N, Genistein, United States Pharmacopeia (USP) Reference Standard, Genistein, Pharmaceutical Secondary Standard; Certified Reference Material, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
ID: 6
InChIKey: OHCQJHSOBUTRHG-KGGHGJDLSA-N SMILES: CC(=O)O[C@H]1[C@H]([C@@H]2[C@]([C@H](CCC2(C)C)O)([C@@]3([C@@]1(O[C@@](CC3=O)(C)C=C)C)O)C)O
biological descriptors:
CFTR relevance: CFTR activatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
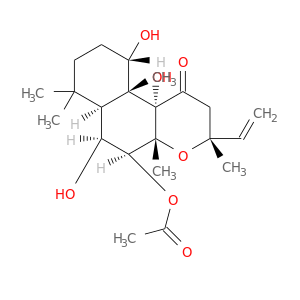
CID is 47936
synonyms found at PubChem are:
forskolin, Colforsin, 66575-29-9, Coleonol, colforsina, colforsine, colforsinum, Boforsin, Colforsine [French], Colforsinum [Latin], Colforsina [Spanish], Coleonolk, Colforsin [USAN:INN], UNII-1F7A44V6OU, 7beta-Acetoxy-8,13-epoxy-1alpha,6beta,9alpha-trihydroxylabd-14-en-11-one, EINECS 266-410-9, HL 362, NSC 357088, NSC 375489, Forskolin, Coleus forskohlii, CHEMBL52606, 1F7A44V6OU, (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxododecahydro-1H-benzo[f]chromen-5-yl acetate, L 75 1362B, CHEBI:42471, HL-362, NSC375489, 64657-11-0, NCGC00024996-02, L-751362B, 7-beta-Acetoxy-8,13-epoxy-1-alpha,6-beta,9-alpha-trihydroxylabd-14-en-11-one, DSSTox_CID_20484, DSSTox_RID_79500, DSSTox_GSID_40484, Forsklin, (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-Dodecahydro-5,6,10,10b-tetrahydroxy-3,4a,7,7,10a-pentamethyl-3-vinyl-1H-naphtho(2,1-b)pyran-1-one 5-acetate, 66428-89-5, C22H34O7, ForsLean, 7beta-Acetoxy-8,13-epoxy-1alpha,6beta,9alpha-trihydroxy-labd-14-en-11-one, (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-3-vinyldodecahydro-1H-benzo[f]chromen-5-yl acetate, 1H-Naphtho(2,1-b)pyran-1-one, 5-(acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-, (3R-(3alpha,4abeta,5beta,6beta,6aalpha,10alpha,10abeta,10balpha))-, 1H-Naphtho(2,1-b)pyran-1-one, dodecahydro-5-(acetyloxy)-3-ethenyl-3,4a,7,7,10a-pentamethyl-6,10,10b-trihydroxy-, (3R-(3-alpha,4a-beta,5-beta,6-beta,6a-alpha,10-alpha,10a-beta,10b-alpha))-, 1H-Naphtho[2,1-b]pyran-1-one, 5-(acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-, (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-, FOK, SMR000471881, CAS-66575-29-9, Ocufors, Timsaponin-C, Colforsin (USAN/INN), D0Y7VM, forskolin/ rolipram mixture, MolMap_000021, AC1L2J1R, AC1Q1L8K, SCHEMBL4928, 1H-Naphtho(2,1-b)pyran-1-one, 5-(acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-, cid_47936, MLS001066384, MLS001333255, MLS001333256, MLS002172462, MLS002695942, Forskolin, analytical standard, Forskohlii Root Extract (ForsLean)(20% forskohlin), REGID_for_CID_47936, GTPL5190, DTXSID8040484, BCBcMAP01_000132, OHCQJHSOBUTRHG-KGGHGJDLSA-, AOB6380, BIF1031, MolPort-002-493-717, OHCQJHSOBUTRHG-KGGHGJDLSA-N, ZX-AFC000211, Bio1_000443, Bio1_000932, Bio1_001421, HMS2235C17, HMS3267I16, ZINC3977779, Tox21_110940, Tox21_302399, BDBM50010261, MFCD00082317, s2449, AKOS024456384, Tox21_110940_1, ACN-035218, AM81249, CS-1454, DB02587, LMPR0104030004, PXT0000105, SMP1_000128, NCGC00024996-03, NCGC00024996-04, NCGC00024996-05, NCGC00024996-06, NCGC00177971-03, NCGC00255526-01, 5-(Acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1H-naphtho(2,1-b)pyran-1-one, AS-17443, CC-28833, HY-15371, LS-95557, SC-47275, F0855, FT-0626543, V0151, Y0448, 7088-EP2280000A1, 7088-EP2284169A1, 7088-EP2287147A2, 7088-EP2287161A1, 7088-EP2287162A1, 7088-EP2287165A2, 7088-EP2287166A2, 7088-EP2292620A2, 7088-EP2295550A2, 7088-EP2301931A1, 7088-EP2301937A1, 7088-EP2308562A2, 7088-EP2311806A2, 7088-EP2371811A2, C09076, D03584, MLS-0318096.0001, S-7769, C-21901, SR-01000597378, Forskolin, For use in molecular biology applications, Q-200888, SR-01000597378-1, BRD-K09602097-001-04-5, Forskolin, from Coleus forskohlii, >=98% (HPLC), powder, Forskolin, United States Pharmacopeia (USP) Reference Standard, (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-dodecahydro-1H-naphtho[2,1-b]pyran-5-yl acetate, [(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-5-yl] acetate, [(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-3-vinyl-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-5-yl] acetate, [3R-(3?,4a?,5?,6?,6a?,10?,10a?,10b?)]-5-(Acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1H-naphtho[2,1-b]pyran-1-one, [3R-(3a,4ab,5b,6b,6aa,10a,10ab,10ba)]-5-(Acetyloxy)-3-e thenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1H-naphtho[2,1-b]pyran-1-one, 1H-Naphtho[2,1-b]pyran-1-one,5-(acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-,(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-, 72569-68-7, InChI=1/C22H34O7/c1-8-19(5)11-14(25)22(27)20(6)13(24)9-10-18(3,4)16(20)15(26)17(28-12(2)23)21(22,7)29-19/h8,13,15-17,24,26-27H,1,9-11H2,2-7H3/t13-,15-,16-,17-,19-,20-,21+,22-/m0/s1
ID: 10
InChIKey: OBKXEAXTFZPCHS-UHFFFAOYSA-M SMILES: C1=CC=C(C=C1)CCCC(=O)[O-]
biological descriptors:
CFTR relevance: little or no effectCategory:
Influence on CFTR function likely enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
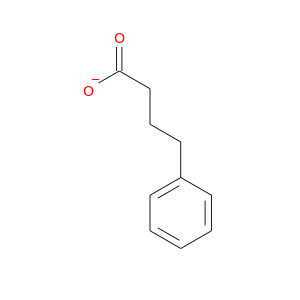
CID is 22053264
synonyms found at PubChem are:
4-phenylbutanoate, 4-Phenylbutyric acid anion, BDBM36184, CHEBI:75317, OBKXEAXTFZPCHS-UHFFFAOYSA-M, AKOS024437455, CJ-00370, ZB001717, J3.540.463E, AB01275463-01, AB01275463_02, A812651
ID: 26
InChIKey: APIXJSLKIYYUKG-UHFFFAOYSA-N SMILES: CC(C)CN1C2=C(C(=O)N(C1=O)C)NC=N2
biological descriptors:
CFTR relevance: induced chloride efflux only in Calu-3 cellsCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Apical membrane & subapical compartment
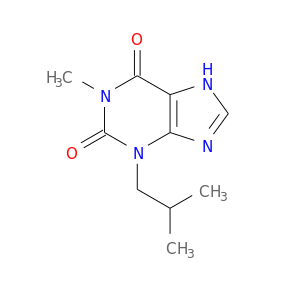
CID is 3758
synonyms found at PubChem are:
3-Isobutyl-1-methylxanthine, IBMX, 28822-58-4, isobutylmethylxanthine, 1-METHYL-3-ISOBUTYLXANTHINE, Methylisobutylxanthine, 3-Isobutyl-1-methyl-1H-purine-2,6(3H,7H)-dione, Xanthine, 3-isobutyl-1-methyl-, 3-Isobutyl-1-methyxanthine, 1H-Purine-2,6-dione, 3,7-dihydro-1-methyl-3-(2-methylpropyl)-, Methyl-isobutylxanthine, 3-Isobutyl 1-methylxanthine, UNII-TBT296U68M, NSC 165960, CCRIS 4290, 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione, CHEBI:34795, 3-isobutyl-1-methyl-7H-xanthine, 3-Isobutyl-1-methyl-3,7-dihydro-1H-purine-2,6-dione, 3-isobutyl-1-methyl-3,9-dihydro-1H-purine-2,6-dione, 2-Acetoxy-Benzoic Acid, 3-Isobutyl-Methylxanthine, EINECS 249-259-3, NSC165960, CHEMBL275084, TBT296U68M, 1-methyl-3-(2-methylpropyl)-3,7-dihydro-1H-purine-2,6-dione, APIXJSLKIYYUKG-UHFFFAOYSA-N, 3-isobutyl-1-methylxanthine (ibmx), IN1293, 3,7-Dihydro-3-isobutyl-1-methyl-1H-purine-2,6-dione, J-640140, 1-methyl-3-(2-methylpropyl)-1,3,7-trihydropurine-2,6-dione, 1H-purine-2,6-dione, 3,9-dihydro-1-methyl-3-(2-methylpropyl)-, 3,7-Dihydro-1-methyl-3-(2-methylpropyl)-1H-purine-2,6-dione, Isobutyltheophylline, IMX, SMR000326697, SR-01000075185, 3 Isobutyl 1 methylxanthine, 1zkl, 1zkn, 3ecn, 3itu, 3jwr, 2hd1, 2r8q, 3qi4, AC1L1GNB, Spectrum2_001705, Spectrum2_001735, Spectrum3_001958, Spectrum4_001052, Spectrum5_001856, Lopac-I-5879, D03ADX, D0GY2T, D0R6MT, MolMap_000030, AC1Q1PM4, Lopac0_000642, Oprea1_135287, Oprea1_228781, SCHEMBL50315, 3-isobutyl-1-methylxantliine, BSPBio_001153, BSPBio_003558, GTPL388, KBioGR_000493, KBioGR_001344, KBioGR_002566, KBioSS_000493, KBioSS_002575, 1-methyl-3-isobutyl-xanthine, MLS001056732, MLS001066424, 3-Isobutyl-1-methyl-xanthine, DivK1c_000922, SPECTRUM1505298, SPECTRUM2300204, SPBio_001690, SPBio_001810, DTXSID0040549, BCBcMAP01_000110, BDBM15336, CHEBI:43253, CTK3J1633, HMS502O04, KBio1_000922, KBio2_000493, KBio2_002566, KBio2_003061, KBio2_005134, KBio2_005629, KBio2_007702, KBio3_000905, KBio3_000906, KBio3_002878, KBio3_003044, KS-00001CXM, 3-isobutyl-1-methyl-9H-xanthine, cMAP_000087, MolPort-001-737-339, MolPort-001-792-510, NINDS_000922, Bio1_000456, Bio1_000945, Bio1_001434, Bio2_000407, Bio2_000887, HMS1362I15, HMS1792I15, HMS1990I15, HMS2090J10, HMS2231C11, HMS3262A05, HMS3369E16, HMS3403I15, HMS3604D14, HMS3648O18, ALBB-024315, ZINC3861807, Tox21_500642, 3-ISOBUTHYL-1-METHYLXANTHINE, ANW-54495, BDBM50027176, BS0316, CCG-39513, CCG-39624, GL2425, HSCI1_000261, MFCD00005584, PDSP1_000324, PDSP2_000322, SC2964, AKOS003390599, AKOS015903085, CS-3361, DB07954, LP00642, MCULE-3854999514, NSC-165960, SC 2964, VZ21530, IDI1_000922, IDI1_002162, NCGC00015559-01, NCGC00015559-02, NCGC00015559-03, NCGC00015559-04, NCGC00015559-05, NCGC00015559-06, NCGC00015559-07, NCGC00015559-08, NCGC00015559-09, NCGC00015559-10, NCGC00015559-11, NCGC00094009-01, NCGC00094009-02, NCGC00094009-03, NCGC00094009-04, NCGC00094009-05, NCGC00094009-06, NCGC00261327-01, Xanthine, 1-methyl-3-(2-methylpropyl), AJ-46242, AK-84450, BC219667, HY-12318, SC-84962, ST055758, IBMX(NSC165960; SC2964), 3-isobutyl-1-methyl-7H-purine-2,6-dione, AX8087568, DB-047466, LS-162537, [Eur J Pharmacol 170: 35 (1989)], B7206, EU-0100642, FT-0615920, R4973, WLN: T56 BM DN FNVNVJ F1Y1&1 H1, 3-Isobutyl-1-methyl-2,6(1H,3H)-purinedione, C13708, I 5879, 3-Isobutyl-1-methylxanthine, BioUltra, >=99%, 822I584, L001156, 3-Isobutyl-1-methyl-3,7-dihydro-purine-2,6-dione, 3-Isobutyl-1-methyl-3,9-dihydro-purine-2,6-dione, J-800144, SR-01000075185-1, SR-01000075185-6, 3-Isobutyl-1-methylxanthine, >=99% (HPLC), powder, BRD-K94979336-001-06-9, BRD-K94979336-001-09-3, I14-18716, 1H-Purine-2, 3,7-dihydro-1-methyl-3-(2-methylpropyl)-, 1-methyl-3-(2-methylpropyl)-3,9-dihydro-1H-purine-2,6-dione, 1-methyl-3-(2-methylpropyl)-2,3,6,7-tetrahydro-1H-purine-2,6-dione