ID: 1
InChIKey: TZBJGXHYKVUXJN-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment several
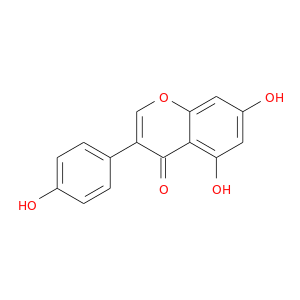
CID is 5280961
synonyms found at PubChem are:
genistein, 446-72-0, Prunetol, Genisteol, 4',5,7-Trihydroxyisoflavone, Genisterin, Sophoricol, 5,7,4'-Trihydroxyisoflavone, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one, Bonistein, Genestein, Differenol A, NPI 031L, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, C.I. 75610, SIPI 807-1, 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one, UNII-DH2M523P0H, NSC 36586, 4,5,7-Trihydroxyiso-flavone, Lactoferrin-genistein, CCRIS 7675, 4',5, 7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone, NSC36586, CHEMBL44, EINECS 207-174-9, IN1327, BRN 0263823, PTI G4660 (Genistein), ISOFLAVONE, 4',5,7-TRIHYDROXY-, MLS000738127, DH2M523P0H, STO514, CHEBI:28088, PTI-G4660, SIPI-9764-I, TZBJGXHYKVUXJN-UHFFFAOYSA-N, 4′,5,7-Trihydroxyisoflavone, TNP00151, NSC-36586, GENISTEIN (ENDOCRINE DISRUPTER), GEN, NCGC00015479-09, DSSTox_CID_2308, G 6649, G10000, DSSTox_RID_76542, DSSTox_GSID_22308, ENDOCRINE DISRUPTOR (GENISTEIN) (SEE ALSO GENISTEIN (446-72-0)), CAS-446-72-0, SR-01000075498, HSDB 7475, PTI G4660, 3kgt, 3kgu, 4&prime, Genistein, 8, Genistein,(S), PTI-G 4660, Genistein (flavonoid), PubChem9852, Spectrum_000320, Tocris-1110, 1x7r, 2qa8, Genistein 85% HPLC, SpecPlus_000305, AC1NQXT4, Spectrum2_000638, Spectrum3_000678, Spectrum4_001543, Spectrum5_000106, Lopac-G-6649, 4',7-Trihydroxyisoflavone, D0L4FS, MolMap_000022, UPCMLD-DP096, 4,5,7-Trihydroxyisoflavone, 4,6,7-Trihydroxyisoflavone, Isoflavone,5,7-trihydroxy-, Lopac0_000520, Oprea1_224620, Oprea1_437815, SCHEMBL19166, BSPBio_002375, KBioGR_002006, KBioGR_002564, KBioSS_000800, KBioSS_002573, SPECTRUM210296, 5-18-04-00594 (Beilstein Handbook Reference), BIDD:ER0113, DivK1c_006401, Genistein, analytical standard, SPBio_000636, 4',5,7-Trihydroxy isoflavone, 4',5,7-trihydroxy-Isoflavone, GTPL2826, MEGxp0_000568, 4,5,7-Trihydroxy Iso-Flavone, DTXSID5022308, UPCMLD-DP096:001, ACon1_001065, BDBM19459, cid_5280961, KBio1_001345, KBio2_000800, KBio2_002564, KBio2_003368, KBio2_005132, KBio2_005936, KBio2_007700, KBio3_001595, KBio3_003042, AOB5073, CHEBI: 28088, cMAP_000086, MolPort-000-003-911, Bio1_000445, Bio1_000934, Bio1_001423, HMS2271K09, HMS3261H21, HMS3267K14, HMS3428M01, HMS3649B22, HMS3654D17, ACT05962, ALBB-015886, BCP07581, KS-00000MH8, Prunetol solution, 20 mM in DMSO, Tox21_110161, Tox21_201428, Tox21_300585, Tox21_500520, AC-472, BBL010484, CCG-38551, CG-009, Genistein solution, 20 mM in DMSO, LMPK12050218, MFCD00016952, s1342, SBB066115, STK801619, ZINC18825330, AKOS001590147, Tox21_110161_1, CS-1534, DB01645, KS-5128, LP00520, LS-1266, MCULE-4857649752, RL03694, RP29616, SMP1_000133, Genistein; 4',5,7-Trihydroxyisoflavone, NCGC00015479-01, NCGC00015479-02, NCGC00015479-04, NCGC00015479-05, NCGC00015479-06, NCGC00015479-07, NCGC00015479-08, NCGC00015479-10, NCGC00015479-11, NCGC00015479-12, NCGC00015479-13, NCGC00015479-14, NCGC00015479-15, NCGC00015479-16, NCGC00015479-17, NCGC00015479-18, NCGC00015479-19, NCGC00015479-20, NCGC00025005-01, NCGC00025005-02, NCGC00025005-03, NCGC00025005-04, NCGC00025005-05, NCGC00025005-06, NCGC00025005-07, NCGC00169711-01, NCGC00169711-02, NCGC00254275-01, NCGC00258979-01, NCGC00261205-01, 690224-00-1, AJ-70669, AN-15821, BC200563, HY-14596, KB-52241, NCI60_003369, SC-04581, SMR000112580, ST056352, AB1004490, EU-0100520, FT-0603395, G0272, N1861, Genistein, disposable screening library format, 46G720, C06563, G-2535, Genistein, synthetic, >=98% (HPLC), powder, J10015, K00046, S-7751, US8552057, 2, AB00052696_09, AB00052696_12, A826657, Genistein, primary pharmaceutical reference standard, I06-0431, Q-100484, SR-01000075498-1, SR-01000075498-3, SR-01000075498-6, 3-(4-hydroxyphenyl)-5,7-bis(oxidanyl)chromen-4-one, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone, BRD-K43797669-001-02-3, BRD-K43797669-001-03-1, BRD-K43797669-001-10-6, Genistein, from Glycine max (soybean), ~98% (HPLC), SR-01000075498-10, 5,7-dihydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one, F0001-2388, 4H-1-Benzopyran-4-one,7-dihydroxy-3-(4-hydroxyphenyl)-, UNII-71B37NR06D component TZBJGXHYKVUXJN-UHFFFAOYSA-N, Genistein, United States Pharmacopeia (USP) Reference Standard, Genistein, Pharmaceutical Secondary Standard; Certified Reference Material, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
ID: 41
InChIKey: IYRMWMYZSQPJKC-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O
biological descriptors:
CFTR relevance: influence of intracellular CFTR distributionCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
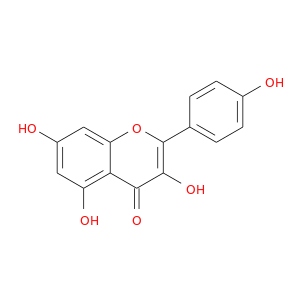
CID is 5280863
synonyms found at PubChem are:
kaempferol, 520-18-3, Kempferol, Kaempherol, Populnetin, Rhamnolutein, Robigenin, Trifolitin, Pelargidenolon, Rhamnolutin, Swartziol, 3,4',5,7-Tetrahydroxyflavone, Indigo Yellow, Kampherol, Campherol, Kampferol, Nimbecetin, Kaemferol, 5,7,4'-Trihydroxyflavonol, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, Pelargidenolon 1497, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, C.I. 75640, CCRIS 41, NSC 407289, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one, 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-, Flavone, 3,4',5,7-tetrahydroxy-, NSC 656277, UNII-731P2LE49E, EINECS 208-287-6, CHEMBL150, BRN 0304401, AI3-36096, CHEBI:28499, IYRMWMYZSQPJKC-UHFFFAOYSA-N, 731P2LE49E, 3,4′,5,7-Tetrahydroxyflavone, NSC656277, NSC-407289, NSC-656277, CAS-520-18-3, DSSTox_CID_768, DSSTox_RID_75781, DSSTox_GSID_20768, Q-100584, SMR000112585, Pelargidenon, Kampcetin, 3,5,7,4'-Tetrahydroxyflavone, HSDB 7703, 4det, Kaempferol,(S), AC1NQXP1, 5,4'-Trihydroxyflavonol, Prestwick0_001098, Prestwick1_001098, Prestwick2_001098, Prestwick3_001098, D0G3TK, 3,5,7-Tetrahydroxyflavone, 4',5,7-trihydroxyflavonol, BIDD:PXR0073, Oprea1_650954, SCHEMBL18817, BSPBio_001176, 5-18-05-00251 (Beilstein Handbook Reference), MLS000697730, MLS001055391, MLS001074884, MLS006010737, BIDD:ER0134, SPBio_003058, 3,4,5,7-Tetrahydroxyflavone, Kaempferol, analytical standard, BDBM7462, BPBio1_001294, MEGxp0_001283, DTXSID7020768, Flavone,4',5,7-tetrahydroxy-, ACon1_001867, cid_5280863, CHEBI: 28499, MolPort-001-741-568, HMS1571K18, HMS2098K18, HMS2267I09, HMS3656M03, KAEMPFEROL ROBIGENIN; 3,4',5,7-TETRAHYDRO-XY-FLAVONE, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1- benzopyran-4-one, Kaempferol, >=97.0% (HPLC), TNP00039, ZINC3869768, Tox21_201165, Tox21_303363, AC-544, CK0011, GP7425, HSCI1_000027, LMPK12110003, LS-176, MFCD00016938, NSC407289, s2314, SBB066091, Kaempferol solution, 20 mM in DMSO, AKOS015895240, Kaempferol, >=90% (HPLC), powder, CCG-202823, CS-1273, DB01852, GS-3570, MCULE-8965218413, NCGC00016480-01, NCGC00016480-02, NCGC00016480-03, NCGC00016480-04, NCGC00016480-05, NCGC00016480-06, NCGC00016480-07, NCGC00016480-09, NCGC00091036-01, NCGC00091036-02, NCGC00164322-01, NCGC00179275-01, NCGC00179275-02, NCGC00257464-01, NCGC00258717-01, AN-15750, BC215517, CC-29746, HY-14590, KB-79581, SC-17291, ST030560, AB0010534, TR-018501, AB00514046, FT-0614420, K0018, N1719, W1682, 3,4',5,7-tetrahydroxy-Flavone (7CI,8CI), C05903, J10449, Kaempferol, disposable screening library format, S00111, W-2776, Flavone, 3,4',5,7-tetrahydroxy- (7CI,8CI), 520K183, A828886, C-18018, SR-01000765646, I06-0240, Kaempferol, primary pharmaceutical reference standard, SR-01000765646-3, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-chromen-4-one, BRD-K12807006-001-05-2, BRD-K12807006-001-10-2, 2-(4-hydroxyphenyl)-3,5,7-tris(oxidanyl)chromen-4-one, 3,4',5,7-tetrahydroxyflavone solution, 20 mM in DMSO, A91A6666-86C8-4B33-B3EF-F74CD3CD7F47, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1-benzopyran-4-one, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #, 3,5,7-Trihydroxy-2-[4-hydroxy-phenyl]-4H-1-benzopyran-4-one, 4H-1-Benzopyran-4-one,3,5,7-trihydroxy-2-(4-hydroxyphenyl)-, 4H-1-Benzopyran-4-one,5,7-trihydroxy-2-(4-hydroxyphenyl)-, Kaempferol, United States Pharmacopeia (USP) Reference Standard, 3,5,7-Trihydroxy-2-[4-hydroxy- phenyl]-4H-1-benzopyran-4-one, 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)- (9CI), 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one solution, 20 mM in DMSO, 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-5,7,4'-Trihydroxyflavonol
ID: 9
InChIKey: KZNIFHPLKGYRTM-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O
biological descriptors:
CFTR relevance: inactiveCategory:
Influence on CFTR function inconsistent assignment
Order of interaction unknown
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
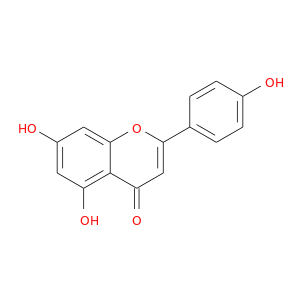
CID is 5280443
synonyms found at PubChem are:
apigenin, 520-36-5, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, Chamomile, Spigenin, Versulin, Apigenol, 4',5,7-Trihydroxyflavone, Apigenine, C.I. Natural Yellow 1, 5,7,4'-Trihydroxyflavone, Pelargidenon 1449, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone, 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone, UCCF 031, NSC 83244, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, UNII-7V515PI7F6, 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one, CCRIS 3789, CHEBI:18388, CHEMBL28, EINECS 208-292-3, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-, BRN 0262620, FLAVONE, 4',5,7-TRIHYDROXY-, 4′,5,7-Trihydroxyflavone, KZNIFHPLKGYRTM-UHFFFAOYSA-N, 7V515PI7F6, NSC83244, NSC-83244, CAS-520-36-5, ST056301, DSSTox_CID_2391, DSSTox_RID_76568, DSSTox_GSID_22391, Q-100586, Q-200822, SMR000326850, SR-01000075663, Chamomile Powder, HSDB 7573, 4der, 4dgm, 4hkk, Naringenin, 18, Prestwick_719, Apigenin, 13, PubChem9831, Tocris-1227, 3cf9, 4',7-Trihydroxyflavone, BiomolKI_000078, Prestwick0_000414, Prestwick1_000414, Prestwick2_000414, Prestwick3_000414, Spectrum2_000428, Spectrum3_001882, Spectrum4_001999, Lopac-A-3145, BiomolKI2_000082, D00RIX, 4,5, 7-Trihydroxyflavone, AC1NQX15, Lopac0_000065, Oprea1_622293, SCHEMBL19428, 4',5,7-trihydroxy-Flavone, Apigenin, analytical standard, BSPBio_000368, BSPBio_003384, KBioGR_002565, SPECTRUM200846, 5-18-04-00574 (Beilstein Handbook Reference), MLS000697626, MLS000859991, MLS001074874, MLS006011839, BIDD:ER0135, DivK1c_000798, SCHEMBL222227, SPBio_000416, SPBio_002307, ghl.PD_Mitscher_leg0.1194, BDBM7458, BPBio1_000406, GTPL4136, MEGxp0_000176, DTXSID6022391, ACon1_002450, cid_5280443, HMS502H20, KBio1_000798, KBio3_002887, BIK9018, OR7265T, MolPort-001-740-354, NINDS_000798, ZX-AFC000435, Bio1_000376, Bio1_000865, Bio1_001354, HMS1569C10, HMS1922P22, HMS2096C10, HMS2230D17, HMS3260M11, HMS3267D21, HMS3373B18, HMS3561P09, HMS3655D18, Apigenin, >=95.0% (HPLC), 4',5,7-Trihydroxyflavone, 97%, ACN-S003241, BCP28288, HY-N1201, ZINC3871576, ZX-AT019281, Tox21_201542, Tox21_302884, Tox21_500065, Apigenin; 4',5,7-Trihydroxyflavone, BBL010499, BS0030, CCG-40061, GP1532, HSCI1_000221, LMPK12110005, MFCD00006831, s2262, SBB066087, STK801630, AKOS002140699, AC-8011, ACN-034762, AN-8432, CS-5432, DB07352, EBD2138579, LP00065, LS-2209, MCULE-6141069907, ND-9076, SDCCGMLS-0066379.P001, TRA0067512, IDI1_000798, SMP2_000338, Apigenin, >=97% (TLC), from citrus, NCGC00015049-01, NCGC00015049-02, NCGC00015049-03, NCGC00015049-04, NCGC00015049-05, NCGC00015049-06, NCGC00015049-07, NCGC00015049-08, NCGC00015049-09, NCGC00015049-10, NCGC00015049-11, NCGC00015049-12, NCGC00015049-13, NCGC00015049-14, NCGC00015049-15, NCGC00015049-16, NCGC00015049-18, NCGC00025057-01, NCGC00025057-02, NCGC00025057-03, NCGC00025057-04, NCGC00025057-05, NCGC00025057-06, NCGC00025057-07, NCGC00025057-08, NCGC00025057-09, NCGC00169835-01, NCGC00169835-02, NCGC00169835-03, NCGC00256419-01, NCGC00259092-01, NCGC00260750-01, 4CN-0925, AJ-46351, AK-88794, CC-24158, CJ-10995, KB-78227, NCI60_041830, SC-05011, SY005957, TS-00897, AB0010536, AB1011450, AX8015784, LY 080400, ST2411642, TC-307820, TR-018510, EU-0100065, FT-0622445, FT-0623582, N1828, 20A365, A 3145, C01477, J10341, K00045, M-6923, Apigenin, >=97% (TLC), from parsley, powder, Biochem Biophys Res Comm 212: 767 (1997), 4 inverted exclamation marka,5,7-Trihydroxyflavone, 5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one, Apigenin, primary pharmaceutical reference standard, C-16977, 4 inverted exclamation mark ,5,7-trihydroxyflavone, I06-0221, SR-01000075663-1, SR-01000075663-3, SR-01000075663-7, SR-01000075663-8, BRD-K01493881-001-10-4, BRD-K01493881-001-17-9, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #, 4H-1-Benzopyran-4-one,7-dihydroxy-2-(4-hydroxyphenyl)-, D50A2D8A-6D8B-4708-B21E-2DE9580D033F, Apigenin, United States Pharmacopeia (USP) Reference Standard, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)- (9CI), 461015-54-3, 8002-66-2
ID: 18
InChIKey: REFJWTPEDVJJIY-UHFFFAOYSA-N SMILES: C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
biological descriptors:
CFTR relevance: CFTR activatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
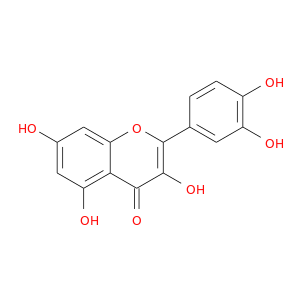
CID is 5280343
synonyms found at PubChem are:
quercetin, 117-39-5, Meletin, Sophoretin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, 3,3',4',5,7-Pentahydroxyflavone, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, Flavin meletin, Cyanidelonon 1522, T-Gelb bzw. grun 1, 3,5,7,3',4'-Pentahydroxyflavone, C.I. Natural Yellow 10, Quercetin content, Kvercetin, Quertin, C.I. Natural red 1, Kvercetin [Czech], Natural Yellow 10, C.I. 75670, CI Natural Yellow 10, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, 3',4',5,7-Tetrahydroxyflavan-3-ol, 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, C.I. Natural yellow 10 & 13, CCRIS 1639, HSDB 3529, Flavone, 3,3',4',5,7-pentahydroxy-, NCI-C60106, UNII-9IKM0I5T1E, NSC 9219, 3',4',5,7-tetrahydroxyflavon-3-ol, 3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-on, Cyanidenolon 1522, CHEBI:16243, AI3-26018, NSC9219, CHEMBL50, EINECS 204-187-1, MixCom3_000183, BRN 0317313, C.I . natural yellow 10, 9IKM0I5T1E, CI 75670, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one, 2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one, REFJWTPEDVJJIY-UHFFFAOYSA-N, KUC104418N, KUC107684N, 3,3',4,5,7-Pentahydroxyflavone, LIM-5662, LNS-5662, NSC-9219, TNP00070, TNP00089, KSC-23-76, KSC-10-126, P0042, DSSTox_CID_1218, Q 0125, DSSTox_RID_76017, DSSTox_GSID_21218, 74893-81-5, QUE, CU-01000012502-3, Q-200333, BRD9794, 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one, BRD-9794, CAS-117-39-5, NSC57655, NSC58588, SR-01000076098, Ritacetin, Quer, 4dfu, 4mra, Quercetin_sathishkumar, Quercetin (Sophoretin), Quercetin - Sophoretin, Spectrum_000124, Tocris-1125, 3cf8, AC1NQWX8, BiomolKI_000062, 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, zirconium(2+) salt (1:1), Maybridge1_008992, Prestwick0_000507, Prestwick1_000507, Prestwick2_000507, Prestwick3_000507, Spectrum2_000059, Spectrum3_000642, Spectrum4_000807, Spectrum5_001389, Lopac-Q-0125, C.I. natural yellow 13, BiomolKI2_000068, D0K8KX, Enicostemma Littorale Blume, UPCMLD-DP081, NCIOpen2_007628, NCIOpen2_007882, BIDD:PXR0007, Lopac0_000999, SCHEMBL19723, BSPBio_000433, BSPBio_001068, BSPBio_002243, KBioGR_000408, KBioGR_001293, KBioSS_000408, KBioSS_000584, 5-18-05-00494 (Beilstein Handbook Reference), KSC497C4F, MLS006011766, BIDD:ER0315, DivK1c_000485, SCHEMBL219729, SPECTRUM1500672, SPBio_000217, SPBio_002354, AC1Q795S, AC1Q795T, BDBM7460, BPBio1_000477, GTPL5346, MEGxp0_000381, SGCUT00001, 3,4',5,7-Pentahydroxyflavone, DTXSID4021218, UPCMLD-DP081:001, ACon1_000560, CTK3J7142, HMS501I07, KBio1_000485, KBio2_000408, KBio2_000584, KBio2_002976, KBio2_003152, KBio2_005544, KBio2_005720, KBio3_000775, KBio3_000776, KBio3_001463, 3,7,3',4'-Pentahydroxyflavone, MolPort-001-740-557, NINDS_000485, 3',5,7-Tetrahydroxyflavan-3-ol, Bio1_000369, Bio1_000858, Bio1_001347, Bio2_000374, Bio2_000854, HMS1362F09, HMS1792F09, HMS1923O19, HMS1990F09, HMS3263G19, HMS3267M12, HMS3649D04, HMS3656C15, to_000078, ZINC3869685, 3,5,7,3',4'-Pentahydroxyflavon, Tox21_202308, Tox21_300285, Tox21_500999, ANW-73134, BBL005513, BS0155, CCG-40054, CQ0011, Flavone,3',4',5,7-pentahydroxy-, GP9232, LMPK12110004, LS-589, MFCD00006828, NSC324608, Quercetin solution, 20 mM in DMSO, s2391, SBB012521, STK365650, Quercetin, >=95% (HPLC), solid, 3,4',5,5',7-pentahydroxy-Flavone, AKOS000511724, CS-3981, DB04216, DS-3416, EBD2197934, LP00999, MCULE-2433372790, NUT0000107, RTX-012622, IDI1_000485, IDI1_002129, KS-0000021G, SMP1_000252, Flavone, 3,4',5,5',7-pentahydroxy-, NCGC00015870-01, NCGC00015870-02, NCGC00015870-03, NCGC00015870-05, NCGC00015870-06, NCGC00015870-07, NCGC00015870-08, NCGC00015870-09, NCGC00015870-10, NCGC00015870-11, NCGC00015870-12, NCGC00015870-13, NCGC00015870-14, NCGC00015870-15, NCGC00015870-16, NCGC00015870-17, NCGC00015870-18, NCGC00015870-19, NCGC00015870-21, NCGC00015870-22, NCGC00015870-23, NCGC00015870-24, NCGC00025016-01, NCGC00025016-02, NCGC00025016-03, NCGC00025016-04, NCGC00025016-05, NCGC00025016-06, NCGC00025016-07, NCGC00025016-08, NCGC00168962-01, NCGC00168962-02, NCGC00168962-03, NCGC00168962-04, NCGC00254218-01, NCGC00259857-01, NCGC00261684-01, 4CN-0923, AC-19596, AC-29756, AJ-46321, AK106169, AN-22768, BAS 00649429, CJ-10980, HY-18085, KB-66753, LS-69030, NCI60_042036, S295, SC-25667, SMR000112559, ST024706, ST057237, Quercetin, Sophoretin, Meletin, Quercetine, AX8030401, KB-221421, EU-0100999, FT-0603318, FT-0655108, N1841, Q0025, ST24039236, Quercetin, disposable screening library format, Quercetin; 3,3',4',5,7-Pentahydroxyflavone, 17Q395, A-8821, C00389, K00029, S00057, WLN: T66 BO EVJ CR CQ DQ & DQ GQ IQ, SR-01000076098-1, SR-01000076098-3, SR-01000076098-7, SR-01000076098-8, BRD-K97399794-001-02-1, BRD-K97399794-001-07-0, BRD-K97399794-001-09-6, BRD-K97399794-001-11-2, BRD-K97399794-335-03-1, SR-01000076098-11, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate, 3,3',4',5,7-pentahydroxyflavone solution, 20 mM in DMSO, A1784/0075599, 49643640-FD4C-4B93-BD28-0D7C2021CC52, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one #, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one solution, 20 mM in DMSO, 73123-10-1
ID: 10
InChIKey: OBKXEAXTFZPCHS-UHFFFAOYSA-M SMILES: C1=CC=C(C=C1)CCCC(=O)[O-]
biological descriptors:
CFTR relevance: little or no effectCategory:
Influence on CFTR function likely enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
CID is 22053264
synonyms found at PubChem are:
4-phenylbutanoate, 4-Phenylbutyric acid anion, BDBM36184, CHEBI:75317, OBKXEAXTFZPCHS-UHFFFAOYSA-M, AKOS024437455, CJ-00370, ZB001717, J3.540.463E, AB01275463-01, AB01275463_02, A812651