ID: 3
InChIKey: FJNFVCAHHFNREI-UHFFFAOYSA-N SMILES: CC(C1=NC2=CC=CC=C2C(=N1)OC3CCCCC3)N4CCN(CC4)S(=O)(=O)C5=CC=C(C=C5)OC
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Apical membrane & subapical compartment
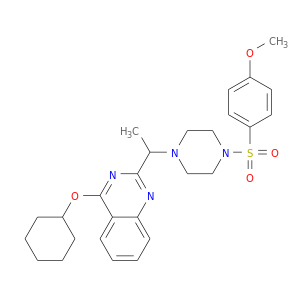
CID is 11957831
synonyms found at PubChem are:
815592-21-3, 4-(cyclohexyloxy)-2-(1-(4-[(4-methoxybenzene)sulfonyl]piperazin-1-yl)ethyl)quinazoline, CFcor-325, VRT-325, 4-(Cyclohexyloxy)-2-(1-(4-(4-methoxyphenylsulfonyl)piperazin-1-yl)ethyl)quinazoline, 4-(cyclohexyloxy)-2-(1-{4-[(4-methoxybenzene)sulfonyl]piperazin-1-yl}ethyl)quinazoline, ACMC-20p1e2, GTPL4340, SCHEMBL3822809, VRT325, CHEMBL1257047, CTK9A5799, DTXSID00474707, VRT 325, MolPort-006-415-015, KS-000000GF, AKOS030228615, 4-cyclohexyloxy-2-[1-[4-(4-methoxyphenyl)sulfonylpiperazin-1-yl]ethyl]quinazoline, KB-01359, KB-34900, A1-01884, 4-(Cyclohexyloxy)-2-(1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)ethyl)quinazoline, 4-(cyclohexyloxy)-2-(1-{4-[(4-methoxyphenyl)sulfonyl]-1-piperazinyl}ethyl)quinazoline
ID: 2940
InChIKey: JNAHVYVRKWKWKQ-CYBMUJFWSA-N SMILES: C[C@@]1(CCCN1)C2=NC3=C(C=CC=C3N2)C(=O)N
biological descriptors:
CFTR relevance: PARP inhibitorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Nucleus (Transcription)
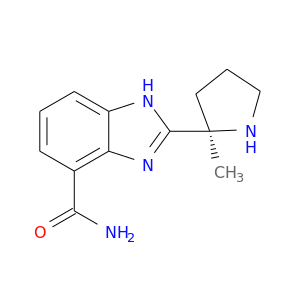
CID is 11960529
synonyms found at PubChem are:
Veliparib, 912444-00-9, ABT-888, ABT 888, Veliparib (ABT-888), ABT-888 (Veliparib), ABT888, UNII-01O4K0631N, 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide, CHEBI:62880, 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide, (R)-2-(2-methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-4-carboxamide, 01O4K0631N, 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benimidazole-4-, Veliparib dihydrochloride, 2-((2r)-2-methyl-2-pyrrolidinyl)-1h-benzimidazole-7-carboxamide, (2r)-2-(7-Carbamoyl-1h-Benzimidazol-2-Yl)-2-Methylpyrrolidinium, (R)-2-(2-methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-7-carboxamide, 2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazole-4-carboxamide, ABT-888(Veliparib), NSC-737664, Veliparib [USAN:INN], 2-((2R)-2-methylpyrrolidin-2-yl)-1H-benzimidazole-4-carboxamide, 2-[(2R)-2-methyl-2-pyrrolidinyl]-1H-benzimidazole-7-carboxamide, 78P, ABT-888 Veliparib, ABT888 (free base), D0Q7UD, A861695, benzimidazole carboxamide, 3a, MLS006010184, Veliparib (JAN/USAN/INN), SCHEMBL422318, CHEMBL506871, GTPL7417, QCR-33, BDBM27135, ABT-695, DTXSID90238456, EX-A001, MolPort-016-633-168, BDBM209932, AOB87114, EBD52357, 1H-Benzimidazole-7-carboxamide, 2-[(2R)-2-methyl-2-pyrrolidinyl]-, ABP000419, AN-034, BN0721, IN2264, NSC737664, s1004, ZINC84610155, AKOS015951440, AKOS017343746, API0024782, CS-0076, DB07232, EX-7209, RL05736, ABT-888(Veliparib)/MX-1,ABT888, NCGC00250404-01, AC-23330, AN-26402, AS-19397, BC623040, EN002695, HY-10129, KB-67929, SMR004701290, ABT-888 (Veliparib, NSC 737664), AB1010175, FT-0660949, X7540, A24888, D09692, W-5661, A-861695, J-505211, BRD-K87142802-001-02-7, Veliparib;ABT-888;ABT888;ABT 888;912444-00-9, 1H-Benzimidazole-4-carboxamide, 2-((2R)-2-methyl-2-pyrrolidinyl)-, 2-[(2R)-2-methylpyrrolidin-2-yl]-3H-1,3-benzodiazole-4-carboxamide, (R)-2-(2-Methylpyrrolidin-2-yl)-1H-benimidazole-4-carboxamide (VELIPARIB), ABT-888;2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benimidazole-4- carboxamide;(R)-2-(2-methylpyrrolidin-2-yl)-3H-benzo[d]imidazole-4-carboxamide
ID: 3014
InChIKey: SFCMPVWUVZLOFH-UHFFFAOYSA-N SMILES: COC(=O)C1=C2C(=NC(=N1)N)CNC(=O)C3=C2C(=C(N3)Br)Br
biological descriptors:
CFTR relevance: CFTR Trafficking Corrector, PARP inhibitorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
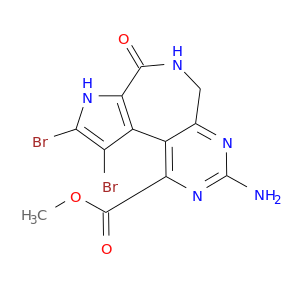
CID is 25168132
synonyms found at PubChem are:
SCHEMBL13042346, 1,2-Dibromo-4-oxo-8-amino-3,4,5,6-tetrahydro-3,5,7,9-tetraazabenzo[e]azulene-10-carboxylic acid methyl ester
ID: 3061
InChIKey: XQPNCZDPZRCEHB-UHFFFAOYSA-N SMILES: CCOC(=O)C1=C2C(=NC(=N1)N)CNC(=O)C3=C2C(=C(N3)Br)Br
biological descriptors:
CFTR relevance: CFTR Trafficking Corrector, PARP inhibitorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
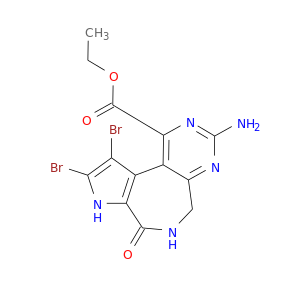
CID is 25168133
synonyms found at PubChem are:
SCHEMBL13042344
ID: 697
InChIKey: GGCJGNBVVMDYKM-UHFFFAOYSA-N SMILES: C1C2=NC(=NC=C2C3=C(C(=O)N1)NC(=C3Br)Br)N
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
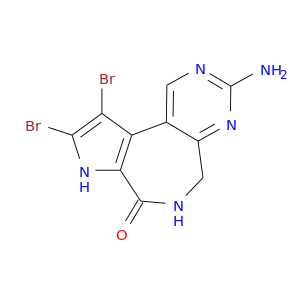
CID is 11440210
synonyms found at PubChem are:
Latonduine A, SCHEMBL13042347
ID: 3029
InChIKey: UYJZZVDLGDDTCL-UHFFFAOYSA-N SMILES: CN(C)CC(=O)NC1=CC2=C(C=C1)NC(=O)C3=CC=CC=C32
biological descriptors:
CFTR relevance: PARP inhibitorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Nucleus (Transcription)
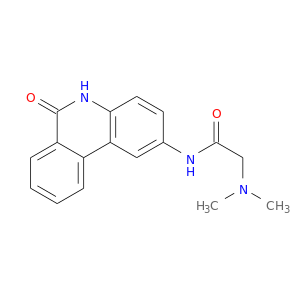
CID is 4858
synonyms found at PubChem are:
PJ34, 344458-19-1, pj-34, CHEMBL372303, UYJZZVDLGDDTCL-UHFFFAOYSA-N, N~2~,N~2~-DIMETHYL-N~1~-(6-OXO-5,6-DIHYDROPHENANTHRIDIN-2-YL)GLYCINAMIDE, P34, 2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridin-2-yl)acetamide, Acetamide, N-(5,6-dihydro-6-oxo-2-phenanthridinyl)-2-(dimethylamino)-, PJ34(free base), 1xk9, D04WDX, SCHEMBL422317, AC1L1J45, ZINC8960, BDBM27497, CTK1B7701, Ibrutinib (PCI32765 pound(c), MolPort-035-395-737, HMS3651B06, BCP07990, 2662AH, HY-13688A, AKOS030229047, CS-1463, DB08348, NCGC00370866-10, AJ-08322, BC600341, DA-42692, AB0109995, FT-0722456, W-5671, BRD-K11853856-003-01-3, 2-(dimethylamino)-N-(6-oxo-5H-phenanthridin-2-yl)acetamide, 2-(dimethylamino)-N-(5,6-dihydro-6-oxophenanthridin-2yl)acetamide, 2-Dimethylamino-N-(6-oxo-5,6-dihydro-phenanthridin-2-yl)-acetamide, N-(6-oxo-5,6-dihydro- phenanthridin-2-yl)-N,N-dimethylacetamide, N-(5,6-Dihydro-6-oxo-2-phenanthridinyl)-2-(dimethylamino)-acetamide