ID: 3
InChIKey: FJNFVCAHHFNREI-UHFFFAOYSA-N SMILES: CC(C1=NC2=CC=CC=C2C(=N1)OC3CCCCC3)N4CCN(CC4)S(=O)(=O)C5=CC=C(C=C5)OC
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Apical membrane & subapical compartment
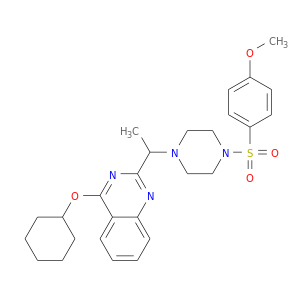
CID is 11957831
synonyms found at PubChem are:
815592-21-3, 4-(cyclohexyloxy)-2-(1-(4-[(4-methoxybenzene)sulfonyl]piperazin-1-yl)ethyl)quinazoline, CFcor-325, VRT-325, 4-(Cyclohexyloxy)-2-(1-(4-(4-methoxyphenylsulfonyl)piperazin-1-yl)ethyl)quinazoline, 4-(cyclohexyloxy)-2-(1-{4-[(4-methoxybenzene)sulfonyl]piperazin-1-yl}ethyl)quinazoline, ACMC-20p1e2, GTPL4340, SCHEMBL3822809, VRT325, CHEMBL1257047, CTK9A5799, DTXSID00474707, VRT 325, MolPort-006-415-015, KS-000000GF, AKOS030228615, 4-cyclohexyloxy-2-[1-[4-(4-methoxyphenyl)sulfonylpiperazin-1-yl]ethyl]quinazoline, KB-01359, KB-34900, A1-01884, 4-(Cyclohexyloxy)-2-(1-(4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)ethyl)quinazoline, 4-(cyclohexyloxy)-2-(1-{4-[(4-methoxyphenyl)sulfonyl]-1-piperazinyl}ethyl)quinazoline
ID: 80
InChIKey: XJGFWWJLMVZSIG-UHFFFAOYSA-N SMILES: C1=CC=C2C(=C1)C(=C3C=CC=CC3=N2)N
biological descriptors:
CFTR relevance: CFTR proteostasis regulatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment several
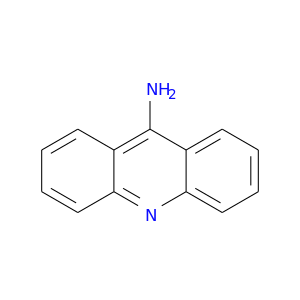
CID is 7019
synonyms found at PubChem are:
9-AMINOACRIDINE, Acridin-9-amine, Aminacrine, 90-45-9, 9-Acridinamine, Aminoacridine, Aminacrin, Izoacridina, Monacrin, Aminoacridina, Aminoacridinum, 10-Amino-5-azaanthracene, 9-Acridinylamine, 9-Aminoakridin, Acridine, 9-amino-, 9-Aminoacridin, 9(10H)-Acridinimine, 9-Aminoakridin [Czech], Aminoacridine [INN:BAN], 9AA, UNII-78OY3Z0P7Z, NSC 13000, CCRIS 748, Acridin-9-ylamine, NSC 7571, EINECS 201-995-6, BRN 0141171, AI3-51012, 78OY3Z0P7Z, 9-AA, CHEBI:74789, XJGFWWJLMVZSIG-UHFFFAOYSA-N, acridine-9-ylamine, Aminopt, Mykocert, 110166-26-2, CS-003/03975023, WLN: T C666 BNJ IZ, MLS000780068, NSC7571, 7AD, 8AD, 9-Aminoacridine hydrochloride hydrate, acridin-9-amine;hydrate;hydrochloride, SMR000420251, iminoacridan, 5-Aminoacridin, 9-amino acridine, 4bds, 9-Amino-acridine, F2179-0009, acridin-9-yl-amine, Quench (Salt/Mix), ACMC-20mczt, Mycosert (Salt/Mix), Spectrum_001108, SpecPlus_000861, 9-Acridinamine (9CI), AC1L1NTJ, Spectrum2_001112, Spectrum3_000617, Spectrum4_000580, Spectrum5_001498, AC1Q4W9J, AC1Q51BO, Acramine Yellow (Salt/Mix), SCHEMBL14999, BSPBio_002154, KBioGR_001020, KBioSS_001588, BIDD:GT0816, CHEMBL43184, DivK1c_006957, SPECTRUM1500810, SPBio_001244, DTXSID2024456, BDBM72700, cid_2723598, CTK0G2217, KBio1_001901, KBio2_001588, KBio2_004156, KBio2_006724, KBio3_001654, KS-00000GTZ, XJGFWWJLMVZSIG-UHFFFAOYSA-, MolPort-001-738-830, HMS1921I16, HMS2092K22, HMS3715H04, Pharmakon1600-01500810, 9-acridinamine;hydrate;hydrochloride, ALBB-020726, BCP25849, HY-B1422, NSC13000, NSC28747, ZX-AN019316, ANW-75188, BBL011755, CCG-39037, NSC-13000, NSC-28747, NSC757794, SBB003606, STK387428, ZINC19014768, AKOS000120447, acridin-9-ylamine;hydrate;hydrochloride, CS-4915, DB11561, LS-1913, MCULE-4734217474, NSC-757794, NCGC00094857-01, NCGC00094857-02, NCGC00094857-03, AJ-45955, AK109135, AS-17405, CC-23490, ST093685, U978, 9-AMINOACRIDINE (5-AMINOACRIDINE), SBI-0051612.P002, AX8014518, DB-026983, KB-250613, TC-163933, A2905, FT-0621612, ST24045903, EN300-17076, M-2337, AB00052180_09, AB00052180_10, C-08694, SR-01000760844, CU-01000012501-2, SR-01000760844-2, BRD-K00535541-001-02-2, BRD-K00535541-311-04-1, 9-AMINO-(N-(2-DIMETHYLAMINO)BUTYL)ACRIDINE-4-CARBOXAMIDE, 9-Aminoacridine, matrix substance for MALDI-MS, >=99.5% (HPLC), 9-AMINOACRIDINE (SEE ALSO 9-AMINOACRIDINE HCL AND 9-AMINOACRIDINE HCL-H2O), 148651-03-0, InChI=1/C13H10N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1-8H,(H2,14,15)
ID: 8
InChIKey: RDOBOPJBMQURAT-UHFFFAOYSA-N SMILES: CC1=C(SC(=N1)NC(=O)C2=CC=CC=C2)C3=CSC(=N3)NC4=C(C=CC(=C4)Cl)OC
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Apical membrane & subapical compartment
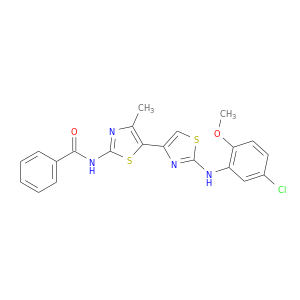
CID is 1144671
synonyms found at PubChem are:
Corr-4a, RDOBOPJBMQURAT-UHFFFAOYSA-N, 421580-53-2, ST075396, N-(2-(5-Chloro-2-methoxyphenylamino)-4'-methyl-4,5'-bithiazol-2'-yl)benzamide, MLS000561131, Oprea1_189688, Oprea1_471654, SCHEMBL837666, CHEMBL260775, DTXSID00360737, MolPort-001-986-142, ZINC888741, AKOS001665304, MCULE-2724995336, BAS 02936954, KB-57187, SMR000156156, EU-0078205, MLS-0094194.0001, SR-01000492559, SR-01000492559-1, N-[2-(5-Chloro-2-methoxy-phenylamino)-4'-methyl-[4,5']bithiazolyl-2' -yl]-benzamide, N-[2-(5-Chloro-2-methoxy-phenylamino)-4'-methyl-[4,5']bithiazolyl-2'-yl]-benzami, N-[2-(5-Chloro-2-methoxyphenylamino)-4'-methyl-[4,5']bithiazolyl-2'-yl]benzamide, N-{2-[(5-chloro-2-methoxyphenyl)amino]-4'-methyl-4,5'-bi-1,3-thiazol-2'-yl}benzamide, N-(5-{2-[(5-chloro-2-methoxyphenyl)amino](1,3-thiazol-4-yl)}-4-methyl(1,3-thia zol-2-yl))benzamide
ID: 21
InChIKey: WAEXFXRVDQXREF-UHFFFAOYSA-N SMILES: C1=CC=C(C=C1)NC(=O)CCCCCCC(=O)NO
biological descriptors:
CFTR relevance: CFTR proteostasis regulatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment several
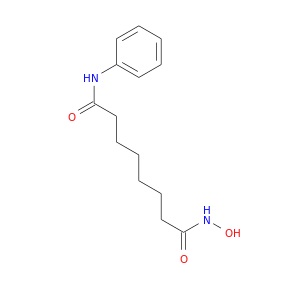
CID is 5311
synonyms found at PubChem are:
Vorinostat, 149647-78-9, SAHA, suberoylanilide hydroxamic acid, Zolinza, N-hydroxy-N'-phenyloctanediamide, Suberanilohydroxamic acid, N1-hydroxy-N8-phenyloctanediamide, SAHA cpd, MK-0683, MK0683, Vorinostat [USAN], Octanediamide, N-hydroxy-N'-phenyl-, CCRIS 8456, Vorinostat (SAHA, MK0683), N-Hyrdroxy-N'-phenyloctanediamide, Vorinostat MSD, UNII-58IFB293JI, Zolinza (TN), OCTANEDIOIC ACID HYDROXYAMIDE PHENYLAMIDE, SHH, N'-hydroxy-N-phenyloctanediamide, CHEMBL98, Vorinostat (JAN/USAN), 58IFB293JI, SKI390, CHEBI:45716, WAEXFXRVDQXREF-UHFFFAOYSA-N, WIN64652, NSC701852, SAHA, Suberoylanilide hydroxamic acid, NSC-701852, NCGC00168085-01, NCGC00168085-02, SW-064652, 8-(hydroxyamino)-8-oxo-N-phenyl-octanamide, Zolinza (TN) (Merck), DSSTox_CID_21133, DSSTox_RID_79632, DSSTox_GSID_41133, N-hydroxy-N'-phenyl-octane-1,8-diotic acid diamide, SMR000486344, NHNPODA, CAS-149647-78-9, SR-05000000373, Vorinostat [USAN:INN], vorinostatum, HSDB 7930, 4lxz, N-Hydroxy-N'-phenyl octanediamide, Zolinza; SAHA, Vorinostat (SAHA), Vorinostat - SAHA, MK 0683, 1zz1, Merck brand of Vorinostat, cc-95, D0E7PQ, AC1L1K2K, cid_5311, SCHEMBL9018, Vorinostat (HDAC inhibitor), Suberoylanilidehydroxamic Acid, MLS001065855, MLS006011941, GTPL6852, Zolinza, MK-0683, SAHA, DTXSID6041133, BDBM19149, CTK8B4125, AOB6083, n-hydroxy-n'-phenyl-octanediamide, QCR-121, SUBERANILOHYDROXAMINIC ACID, SYN3006, 1t69, MolPort-003-850-293, N-hydroxy-N''-phenyloctanediamide, BCPP000018, HMS2219L20, HMS3264D20, HMS3327C12, HMS3650D09, HMS3654G11, HMS3715E22, Pharmakon1600-01502267, BCP01858, KS-00000H3B, N1-hydroxy-N8-phenyl-octanediamide, SAHA, >=98% (HPLC), Vorinostat,SAHA,Zolinza,MK-0683, ZINC1543873, Tox21_112605, Tox21_113623, Vorinostat,CAS:149647-78-9, ANW-43951, MFCD00945317, NSC748799, NSC759852, Octanediamide, N1-hydroxy-N8-phenyl, s1047, SK1390, AKOS015966648, Octanediamide, N1-hydroxy-N8-phenyl-, Tox21_112605_1, AC-1923, AN-5256, CCG-208659, CS-0589, DB02546, DG-0025, MCULE-4234367506, NSC-748799, NSC-759852, RP29421, Suberoylanilide hydroxamic acid (SAHA), NCGC00168085-03, NCGC00168085-04, NCGC00168085-05, NCGC00168085-13, BC677687, BP-30216, HY-10221, KB-60639, SC-20040, AB0009911, LS-186548, LS-186997, LS-187780, RT-015807, AM20030018, FT-0082592, FT-0650593, D06320, K-4759, AB00375377-07, AB00375377-08, AB00375377-09, AB01644613_25, 647S789, P111011, Vorinostat, SAHA, suberoylanilide hydroxamic acid, SR-05000000373-2, SR-05000000373-6, SR-05000000373-8, W-201327, BRD-K81418486-001-01-2, BRD-K81418486-001-10-3, BRD-K81418486-001-12-9, BRD-K81418486-001-13-7, BRD-K81418486-001-17-8, BRD-K81418486-001-18-6, Z1541632802
ID: 2061
InChIKey: SCKYRAXSEDYPSA-UHFFFAOYSA-N SMILES: CC1=CC(=O)N(C(=C1)C2CCCCC2)O
biological descriptors:
CFTR relevance: CFTR proteostasis regulatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment several
CID is 2749
synonyms found at PubChem are:
CICLOPIROX, 29342-05-0, Loprox, Penlac, Ciclopiroxum, Batrafen, HOE 296b, Stieprox, Terit, Ciclopiroxum [INN-Latin], cyclopirox, Mycoster, Ciclopirox gel, cyclopyroxolamine, Loprox cream, HOE-296b, Loprox Gel, Ciclopirox Olamin, Ciclopirox-Olamin, Dafnegin-CSC, 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, 2(1H)-Pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl-, 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-one, Loprox Shampoo, 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone, UNII-19W019ZDRJ, Penlac nail lacquer, Ciclopirox [USAN:BAN:INN], Loprox (TN), Penlac (TN), Ciclopirox (Penlac), Ciclopirox (USP/INN), EINECS 249-577-2, HOE-296, 19W019ZDRJ, 6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one, C12H17NO2, CHEBI:453011, SCKYRAXSEDYPSA-UHFFFAOYSA-N, 2(1H)-Pyridone, 6-cyclohexyl-1-hydroxy-4-methyl-, 6-CYCLOHEXYL-1-HYDROXY-4-METHYL-1H-PYRIDIN-2-ONE, 6-cyclohexyl-1-hydroxy-4-methyl-pyridin-2-one, 6-Cyclohexyl-1-hydroxy-4-methyl-2-(1H)-pyridone, (6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone), W-106995, MLS002153867, CNL8, SMR001233223, Ciclopirox [USAN:USP:INN:BAN], Batrafen (TN), Stieprox (TN), 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one, AC1L1EDN, Prestwick0_000541, Prestwick1_000541, Prestwick2_000541, Prestwick3_000541, Spectrum2_000146, Spectrum3_000351, Spectrum4_000288, Spectrum5_000747, D07GRH, CHEMBL1413, SCHEMBL34424, BSPBio_000581, BSPBio_002041, KBioGR_000816, cid_38911, KSC563C4H, BIDD:GT0080, SPBio_000252, SPBio_002502, BPBio1_000641, ZINC1145, DTXSID9048564, BDBM66087, CTK4G3143, KBio3_001261, MolPort-003-845-943, HMS3656I12, BCP28530, HY-B0450, KS-00000L3N, AN-773, BG0571, s2528, AKOS015895717, AB06517, CS-2561, DB01188, KS-5085, NCGC00017112-04, NCGC00017112-05, NCGC00017112-06, NCGC00017112-08, NCGC00017112-11, NCGC00178850-01, NCGC00178850-02, AC-24195, AJ-07970, AK544043, BC205517, CC-25776, SC-17361, SBI-0206690.P002, AB0011605, AB2000628, KB-270156, LS-174247, TL8002303, 1-hydroxy-4-methyl-6-cyclohexyl-2-pyridone, FT-0602961, 6-cyclohexyl-1-hydroxy-4-methyl-2-pyridinone, D03488, 6-cyclohexyl-4-methyl-1-oxidanyl-pyridin-2-one, AB00053438_09, AB00053438_10, AB00053438_11, 342C050, A819878, C-10474, 6-Cyclohexenyl-1-hydroxy-4-methyl-2(1h)-pyridone, 6-cyclohexyl-1-hydroxy-4-methyl-2(1 H)-pyridinone, Batrafen; Loprox;Mycoster;Stieprox;HOE 296b;Penlac, I06-0862, SR-05000001589-5, 6-cyclohexyl-1-hydroxy-4-methyl-2(1 H )-pyridinone, 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone #, BRD-K13044802-213-04-1, BRD-K13044802-213-09-0, 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methyl-2-pyridone, 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one, 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methyl-2-pyridinone, 2-azanylethanol;6-cyclohexyl-4-methyl-1-oxidanyl-pyridin-2-one