ID: 1
InChIKey: TZBJGXHYKVUXJN-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O
biological descriptors:
CFTR relevance: CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment several
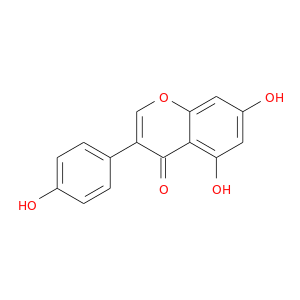
CID is 5280961
synonyms found at PubChem are:
genistein, 446-72-0, Prunetol, Genisteol, 4',5,7-Trihydroxyisoflavone, Genisterin, Sophoricol, 5,7,4'-Trihydroxyisoflavone, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one, Bonistein, Genestein, Differenol A, NPI 031L, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, C.I. 75610, SIPI 807-1, 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one, UNII-DH2M523P0H, NSC 36586, 4,5,7-Trihydroxyiso-flavone, Lactoferrin-genistein, CCRIS 7675, 4',5, 7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone, NSC36586, CHEMBL44, EINECS 207-174-9, IN1327, BRN 0263823, PTI G4660 (Genistein), ISOFLAVONE, 4',5,7-TRIHYDROXY-, MLS000738127, DH2M523P0H, STO514, CHEBI:28088, PTI-G4660, SIPI-9764-I, TZBJGXHYKVUXJN-UHFFFAOYSA-N, 4′,5,7-Trihydroxyisoflavone, TNP00151, NSC-36586, GENISTEIN (ENDOCRINE DISRUPTER), GEN, NCGC00015479-09, DSSTox_CID_2308, G 6649, G10000, DSSTox_RID_76542, DSSTox_GSID_22308, ENDOCRINE DISRUPTOR (GENISTEIN) (SEE ALSO GENISTEIN (446-72-0)), CAS-446-72-0, SR-01000075498, HSDB 7475, PTI G4660, 3kgt, 3kgu, 4&prime, Genistein, 8, Genistein,(S), PTI-G 4660, Genistein (flavonoid), PubChem9852, Spectrum_000320, Tocris-1110, 1x7r, 2qa8, Genistein 85% HPLC, SpecPlus_000305, AC1NQXT4, Spectrum2_000638, Spectrum3_000678, Spectrum4_001543, Spectrum5_000106, Lopac-G-6649, 4',7-Trihydroxyisoflavone, D0L4FS, MolMap_000022, UPCMLD-DP096, 4,5,7-Trihydroxyisoflavone, 4,6,7-Trihydroxyisoflavone, Isoflavone,5,7-trihydroxy-, Lopac0_000520, Oprea1_224620, Oprea1_437815, SCHEMBL19166, BSPBio_002375, KBioGR_002006, KBioGR_002564, KBioSS_000800, KBioSS_002573, SPECTRUM210296, 5-18-04-00594 (Beilstein Handbook Reference), BIDD:ER0113, DivK1c_006401, Genistein, analytical standard, SPBio_000636, 4',5,7-Trihydroxy isoflavone, 4',5,7-trihydroxy-Isoflavone, GTPL2826, MEGxp0_000568, 4,5,7-Trihydroxy Iso-Flavone, DTXSID5022308, UPCMLD-DP096:001, ACon1_001065, BDBM19459, cid_5280961, KBio1_001345, KBio2_000800, KBio2_002564, KBio2_003368, KBio2_005132, KBio2_005936, KBio2_007700, KBio3_001595, KBio3_003042, AOB5073, CHEBI: 28088, cMAP_000086, MolPort-000-003-911, Bio1_000445, Bio1_000934, Bio1_001423, HMS2271K09, HMS3261H21, HMS3267K14, HMS3428M01, HMS3649B22, HMS3654D17, ACT05962, ALBB-015886, BCP07581, KS-00000MH8, Prunetol solution, 20 mM in DMSO, Tox21_110161, Tox21_201428, Tox21_300585, Tox21_500520, AC-472, BBL010484, CCG-38551, CG-009, Genistein solution, 20 mM in DMSO, LMPK12050218, MFCD00016952, s1342, SBB066115, STK801619, ZINC18825330, AKOS001590147, Tox21_110161_1, CS-1534, DB01645, KS-5128, LP00520, LS-1266, MCULE-4857649752, RL03694, RP29616, SMP1_000133, Genistein; 4',5,7-Trihydroxyisoflavone, NCGC00015479-01, NCGC00015479-02, NCGC00015479-04, NCGC00015479-05, NCGC00015479-06, NCGC00015479-07, NCGC00015479-08, NCGC00015479-10, NCGC00015479-11, NCGC00015479-12, NCGC00015479-13, NCGC00015479-14, NCGC00015479-15, NCGC00015479-16, NCGC00015479-17, NCGC00015479-18, NCGC00015479-19, NCGC00015479-20, NCGC00025005-01, NCGC00025005-02, NCGC00025005-03, NCGC00025005-04, NCGC00025005-05, NCGC00025005-06, NCGC00025005-07, NCGC00169711-01, NCGC00169711-02, NCGC00254275-01, NCGC00258979-01, NCGC00261205-01, 690224-00-1, AJ-70669, AN-15821, BC200563, HY-14596, KB-52241, NCI60_003369, SC-04581, SMR000112580, ST056352, AB1004490, EU-0100520, FT-0603395, G0272, N1861, Genistein, disposable screening library format, 46G720, C06563, G-2535, Genistein, synthetic, >=98% (HPLC), powder, J10015, K00046, S-7751, US8552057, 2, AB00052696_09, AB00052696_12, A826657, Genistein, primary pharmaceutical reference standard, I06-0431, Q-100484, SR-01000075498-1, SR-01000075498-3, SR-01000075498-6, 3-(4-hydroxyphenyl)-5,7-bis(oxidanyl)chromen-4-one, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone, BRD-K43797669-001-02-3, BRD-K43797669-001-03-1, BRD-K43797669-001-10-6, Genistein, from Glycine max (soybean), ~98% (HPLC), SR-01000075498-10, 5,7-dihydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one, F0001-2388, 4H-1-Benzopyran-4-one,7-dihydroxy-3-(4-hydroxyphenyl)-, UNII-71B37NR06D component TZBJGXHYKVUXJN-UHFFFAOYSA-N, Genistein, United States Pharmacopeia (USP) Reference Standard, Genistein, Pharmaceutical Secondary Standard; Certified Reference Material, 4 inverted exclamation marka,5,7-Trihydroxyisoflavone; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
ID: 1471
InChIKey: NGSWKAQJJWESNS-ZZXKWVIFSA-N SMILES: C1=CC(=CC=C1/C=C/C(=O)O)O
biological descriptors:
CFTR relevance: unspecifiedCategory:
Influence on CFTR function unknown
Order of interaction unknown
subcellular compartment unknown
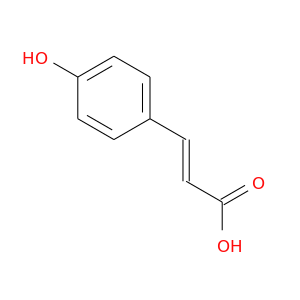
CID is 637542
synonyms found at PubChem are:
p-coumaric acid, 4-Hydroxycinnamic acid, p-Hydroxycinnamic acid, 501-98-4, 4-Coumaric acid, 7400-08-0, trans-4-Hydroxycinnamic acid, trans-p-Coumaric acid, 3-(4-hydroxyphenyl)acrylic acid, p-Cumaric acid, Para-Coumaric acid, Hydroxycinnamic acid, Naringeninic acid, p-Hydroxy-cinnamic acid, p-Hydroxyphenylacrylic acid, (E)-p-Coumaric acid, trans-p-Coumarinic acid, 4'-hydroxycinnamic acid, (E)-3-(4-Hydroxyphenyl)acrylic acid, Cinnamic acid, p-hydroxy-, (E)-p-Hydroxycinnamic acid, trans-p-Hydroxycinnamic acid, 3-(4-Hydroxyphenyl)-2-propenoic acid, (2E)-3-(4-hydroxyphenyl)prop-2-enoic acid, trans-4-coumaric acid, 4-coumarate, 2-propenoic acid, 3-(4-hydroxyphenyl)-, (2E)-, 4-Hydroxycinnamate, 2-Propenoic acid, 3-(4-hydroxyphenyl)-, trans-p-Hydroxycinnamate, 4-hydroxy cinnamic acid, trans-4-hydroxycinnamate, UNII-IBS9D1EU3J, beta-(4-Hydroxyphenyl)acrylic acid, Cinnamic acid, p-hydroxy-, (E)-, (E)-3-(4-Hydroxyphenyl)-2-propenoic acid, Para coumaric acid, 3-(4-hydroxyphenyl)prop-2-enoic acid, (2E)-3-(4-hydroxyphenyl)acrylic acid, trans-p-Cumaric Acid, 4-Hydroxycinamic acid, EINECS 231-000-0, NSC 59260, IBS9D1EU3J, NSC 674321, (E)-4-hydroxycinnamic acid, BRN 2207381, BRN 2207383, CHEMBL66879, C9H8O3, (E)-3-(4-hydroxyphenyl)prop-2-enoic acid, CHEBI:32374, NGSWKAQJJWESNS-ZZXKWVIFSA-N, beta-[4-Hydroxyphenyl]acrylic acid, NSC674321, trans-p-coumarate, 2-Propenoic acid, 3-(4-hydroxyphenyl)-, (E)-, 3-(4-hydroxyphenyl)acrylate, (E)-3-[4-hydroxyphenyl]-2-propenoic acid, 2-Propenoic acid, 3-(4-hydroxyphenyl)-, (Z)-, (E)-3-(4-hydroxyphenyl)prop-2-enoate, CHEBI:36090, hydroxycinnamate, p-Coumaricacid, Para coumarate, 4-coumaric acid, (E)-isomer, p-coumaric-acid, Para-Coumarate, p-Cumarate, p-Hydroxycinnamate, 4qem, 4'-Hydroxycinnamate, 4-Hydroxy cinnamate, p-Coumaric acid,trans, PubChem8247, AC1LCUFZ, PubChem24323, 4f8j, p-Coumaric acid, trans, 4-Hydroxyphenylpropenoate, p-Caumaric acid dehydrogenation homopolymer, bmse000150, bmse000591, bmse010208, D0AU0M, b-[4-Hydroxyphenyl]acrylate, SCHEMBL39106, p-hydroxycinnamic acid (M4), 0-10-00-00297 (Beilstein Handbook Reference), 4-10-00-01005 (Beilstein Handbook Reference), MLS001066419, p-Hydroxycinnamic acid, trans, AKOS BAR-2479, beta-[4-Hydroxyphenyl]acrylate, 2-Propenoic acid, 3-(4-hydroxyphenyl)-, homopolymer, AC1Q5T95, AC1Q71H0, BDBM4374, GTPL5787, P-HYDROXYL CINNAMIC ACID, RARECHEM BK HW 0163, COUMARIC ACID, TRANS-P-, DTXSID6064660, b-[4-Hydroxyphenyl]acrylic acid, TIMTEC-BB SBB007613, ZINC39811, ATTERCOP-CHM AT113965, MolPort-000-860-894, MolPort-004-288-351, HMS1409E10, 3-(4-Hydroxyphenyl)-2-propenoate, AKOS 221-47, BCP22803, HY-N2391, NSC59260, ZX-AT011614, Cinnamic acid, 4-hydroxy-, trans-, AN-287, BBL012226, LABOTEST-BB LT00452637, LABOTEST-BB LT03329617, MFCD00004399, NSC-59260, SBB007613, STL163567, .beta.-[4-Hydroxyphenyl]acrylic acid, AKOS000120685, AS03322, BCP9001042, CS-W020394, DB04066, LS30305, MP-2217, NSC-674321, RP09062, RP17402, RTR-017943, RTR-023948, OTAVA-BB 7020400347, p-Coumaric acid, >=98.0% (HPLC), NCGC00246974-01, 4CN-0926, 50940-26-6, AC-10318, AJ-08911, AS-12000, BP-13278, BR-26304, KB-72564, LS-54111, LS-54112, SC-25929, SC-65982, SMR000112201, ST093691, trans-p-Coumaric acid, analytical standard, AX8022446, ST2402517, TC-164240, TL8005115, TR-017943, TR-023948, AM20050138, N1817, ST24022578, (2E)-3-(4-Hydroxyphenyl)-2-propenoic acid, C00811, (2E)-3-(4-Hydroxyphenyl)-2-propenoic acid #, (E)-3-(4-HYDROXY-PHENYL)-ACRYLIC ACID, 400H080, AE-562/40414679, I01-9546, I01-9648, I04-0102, Q-100560, W-104438, 0C1BFF2D-2CF7-4FC1-9F76-3268C2C7F783, 2-Propenoic acid, 3-(4-hydroxyphenyl)-, (E)- (9CI), F2191-0188, p-Coumaric acid, primary pharmaceutical reference standard, InChI=1/C9H8O3/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-6,10H,(H,11,12)/b6-3
ID: 644
InChIKey: FTVWIRXFELQLPI-CYBMUJFWSA-N SMILES: C1[C@@H](OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
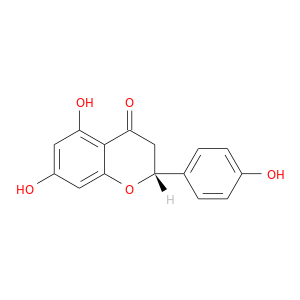
CID is 667495
synonyms found at PubChem are:
(2R)-naringenin, (R)-naringenin, (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, naringenin, (S)-Naringenin, 480-41-1, (+)-(2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone, (-)-Naringenin, 17654-19-2, R-naringenin, (+)-naringenin, AC1LDI7C, ZINC1785, SCHEMBL17166263, CHEBI:50201, MolPort-002-507-277, (2R)-5,7,4'-trihydroxyflavone, HMS3468H18, (2R)-4',5,7-trihydroxyflavanone, ALBB-015405, BBL010488, MFCD03265520, STK801623, AKOS004119880, MCULE-5852778653, (2R)-4',5,7-trihydroxyflavan-4-one, AJ-08090, SC-85987, ZB000410, BB 0261506, N1370, R6691, ST24036200, (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one, 4H-1-benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (2R)-
ID: 9
InChIKey: KZNIFHPLKGYRTM-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O
biological descriptors:
CFTR relevance: inactiveCategory:
Influence on CFTR function inconsistent assignment
Order of interaction unknown
subcellular compartment ER & Golgi (Translation, quality control, trafficking, PTM)
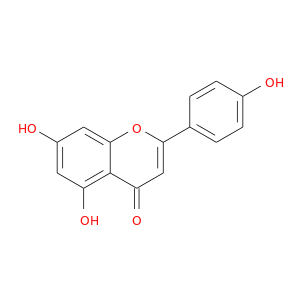
CID is 5280443
synonyms found at PubChem are:
apigenin, 520-36-5, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, Chamomile, Spigenin, Versulin, Apigenol, 4',5,7-Trihydroxyflavone, Apigenine, C.I. Natural Yellow 1, 5,7,4'-Trihydroxyflavone, Pelargidenon 1449, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone, 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone, UCCF 031, NSC 83244, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, UNII-7V515PI7F6, 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one, CCRIS 3789, CHEBI:18388, CHEMBL28, EINECS 208-292-3, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-, BRN 0262620, FLAVONE, 4',5,7-TRIHYDROXY-, 4′,5,7-Trihydroxyflavone, KZNIFHPLKGYRTM-UHFFFAOYSA-N, 7V515PI7F6, NSC83244, NSC-83244, CAS-520-36-5, ST056301, DSSTox_CID_2391, DSSTox_RID_76568, DSSTox_GSID_22391, Q-100586, Q-200822, SMR000326850, SR-01000075663, Chamomile Powder, HSDB 7573, 4der, 4dgm, 4hkk, Naringenin, 18, Prestwick_719, Apigenin, 13, PubChem9831, Tocris-1227, 3cf9, 4',7-Trihydroxyflavone, BiomolKI_000078, Prestwick0_000414, Prestwick1_000414, Prestwick2_000414, Prestwick3_000414, Spectrum2_000428, Spectrum3_001882, Spectrum4_001999, Lopac-A-3145, BiomolKI2_000082, D00RIX, 4,5, 7-Trihydroxyflavone, AC1NQX15, Lopac0_000065, Oprea1_622293, SCHEMBL19428, 4',5,7-trihydroxy-Flavone, Apigenin, analytical standard, BSPBio_000368, BSPBio_003384, KBioGR_002565, SPECTRUM200846, 5-18-04-00574 (Beilstein Handbook Reference), MLS000697626, MLS000859991, MLS001074874, MLS006011839, BIDD:ER0135, DivK1c_000798, SCHEMBL222227, SPBio_000416, SPBio_002307, ghl.PD_Mitscher_leg0.1194, BDBM7458, BPBio1_000406, GTPL4136, MEGxp0_000176, DTXSID6022391, ACon1_002450, cid_5280443, HMS502H20, KBio1_000798, KBio3_002887, BIK9018, OR7265T, MolPort-001-740-354, NINDS_000798, ZX-AFC000435, Bio1_000376, Bio1_000865, Bio1_001354, HMS1569C10, HMS1922P22, HMS2096C10, HMS2230D17, HMS3260M11, HMS3267D21, HMS3373B18, HMS3561P09, HMS3655D18, Apigenin, >=95.0% (HPLC), 4',5,7-Trihydroxyflavone, 97%, ACN-S003241, BCP28288, HY-N1201, ZINC3871576, ZX-AT019281, Tox21_201542, Tox21_302884, Tox21_500065, Apigenin; 4',5,7-Trihydroxyflavone, BBL010499, BS0030, CCG-40061, GP1532, HSCI1_000221, LMPK12110005, MFCD00006831, s2262, SBB066087, STK801630, AKOS002140699, AC-8011, ACN-034762, AN-8432, CS-5432, DB07352, EBD2138579, LP00065, LS-2209, MCULE-6141069907, ND-9076, SDCCGMLS-0066379.P001, TRA0067512, IDI1_000798, SMP2_000338, Apigenin, >=97% (TLC), from citrus, NCGC00015049-01, NCGC00015049-02, NCGC00015049-03, NCGC00015049-04, NCGC00015049-05, NCGC00015049-06, NCGC00015049-07, NCGC00015049-08, NCGC00015049-09, NCGC00015049-10, NCGC00015049-11, NCGC00015049-12, NCGC00015049-13, NCGC00015049-14, NCGC00015049-15, NCGC00015049-16, NCGC00015049-18, NCGC00025057-01, NCGC00025057-02, NCGC00025057-03, NCGC00025057-04, NCGC00025057-05, NCGC00025057-06, NCGC00025057-07, NCGC00025057-08, NCGC00025057-09, NCGC00169835-01, NCGC00169835-02, NCGC00169835-03, NCGC00256419-01, NCGC00259092-01, NCGC00260750-01, 4CN-0925, AJ-46351, AK-88794, CC-24158, CJ-10995, KB-78227, NCI60_041830, SC-05011, SY005957, TS-00897, AB0010536, AB1011450, AX8015784, LY 080400, ST2411642, TC-307820, TR-018510, EU-0100065, FT-0622445, FT-0623582, N1828, 20A365, A 3145, C01477, J10341, K00045, M-6923, Apigenin, >=97% (TLC), from parsley, powder, Biochem Biophys Res Comm 212: 767 (1997), 4 inverted exclamation marka,5,7-Trihydroxyflavone, 5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one, Apigenin, primary pharmaceutical reference standard, C-16977, 4 inverted exclamation mark ,5,7-trihydroxyflavone, I06-0221, SR-01000075663-1, SR-01000075663-3, SR-01000075663-7, SR-01000075663-8, BRD-K01493881-001-10-4, BRD-K01493881-001-17-9, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #, 4H-1-Benzopyran-4-one,7-dihydroxy-2-(4-hydroxyphenyl)-, D50A2D8A-6D8B-4708-B21E-2DE9580D033F, Apigenin, United States Pharmacopeia (USP) Reference Standard, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)- (9CI), 461015-54-3, 8002-66-2
ID: 849
InChIKey: HKEAFJYKMMKDOR-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3C4C(C(C(C(O4)CO)O)O)O)O)O
biological descriptors:
CFTR relevance: unspecifiedCategory:
Influence on CFTR function unknown
Order of interaction unknown
subcellular compartment unknown
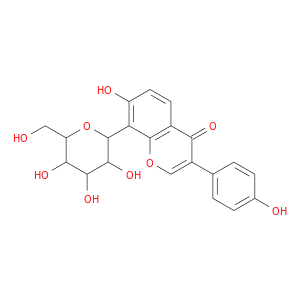
CID is 5385074
synonyms found at PubChem are:
NSC380711, 3681-99-0, Pneranin, 1,5-anhydro-1-[7-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]hexitol, AC1NTOCB, MLS006011792, CHEMBL1319403, SCHEMBL13906069, MolPort-005-943-411, AC-702, BBL030170, STL146384, AKOS005720902, MCULE-8247528856, NSC-380711, PXT0004736, NCGC00096060-01, SMR004703482, ST081391, I06-0429, Hexitol, 1,5-anhydro-1-C-[7-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-8-yl]-, 7-hydroxy-3-(4-hydroxyphenyl)-8-(3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-4H-chromen-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-8-[3,4,5-trihydroxy-6-(hydroxymethyl)(2H-3,4,5,6 -tetrahydropyran-2-yl)]chromen-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-8-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-8-[3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]chromen-4-one
ID: 104
InChIKey: ADFCQWZHKCXPAJ-GFCCVEGCSA-N SMILES: C1[C@H](COC2=C1C=CC(=C2)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: potent ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
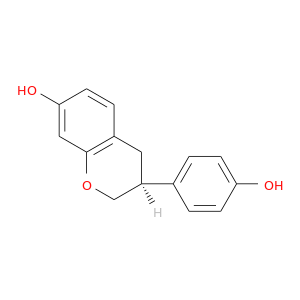
CID is 91469
synonyms found at PubChem are:
Equol, 531-95-3, (S)-3-(4-Hydroxyphenyl)chroman-7-ol, (S)-Equol, S-Equol, (-)-(S)-Equol, (-)-Equol, UNII-2T6D2HPX7Q, 4',7-Isoflavandiol, CCRIS 9222, EINECS 208-522-2, (S)-3,4-Dihydro-3-(4-hydroxyphenyl)-2H-1-benzopyran-7-ol, 2T6D2HPX7Q, CHEMBL198877, CHEBI:34741, (S)-(-)-4',7-Isoflavandiol, (3S)-3-(4-hydroxyphenyl)chroman-7-ol, 4',7-Dihydroxyisoflavan, 2H-1-Benzopyran-7-ol, 3,4-dihydro-3-(4-hydroxyphenyl)-, (3S)-, 3,4-Dihydro-3-(4-hydroxyphenyl)-(S)-2H-1-benzopyran-7-ol, (3S)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol, 7,4'-dihydroxyisoflavan, 7,4'-Isoflavandiol, (3S)-Equol, Equol, (-)-, SE 5OH, AC1L3M4Y, SCHEMBL43647, BIDD:ER0148, DTXSID0022309, AOB5550, AUS 131, AUS-131, ADFCQWZHKCXPAJ-GFCCVEGCSA-N, MolPort-003-847-078, ZINC388661, (3S)-3,4-Dihydro-3-(4-hydroxyphenyl)-2H-1-benzopyran-7-ol, ACT03253, BCP13598, EX-A1354, 2127AH, BDBM50410528, s2450, 4',7-dihydroxy-3,4-dihydroisoflavone, ACN-001462, CS-7937, DB11674, MCULE-5874318771, 7-hydroxy-3-(4'-hydroxyphenyl)chroman, (3s)-3-(4-hydroxyphenyl)-7-chromanol, NCGC00386208-01, AJ-20722, AK-72897, AN-45630, BR-72897, CJ-03317, LS-39394, SC-19277, ZB011640, AX8015938, HY-100583, KB-211349, FT-0649148, ST24026586, C14131, S-2179, 531D953, (3S)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol, UNII-2RZ8A7D0E8 component ADFCQWZHKCXPAJ-GFCCVEGCSA-N, 3,4-dihydro-3-(4- hydroxyphenyl)-(S)-2H-1-benzopyran-7-ol, 20879-01-0
ID: 1175
InChIKey: KKSDGJDHHZEWEP-SNAWJCMRSA-N SMILES: C1=CC(=CC(=C1)O)/C=C/C(=O)O
biological descriptors:
CFTR relevance: unspecifiedCategory:
Influence on CFTR function unknown
Order of interaction unknown
subcellular compartment unknown
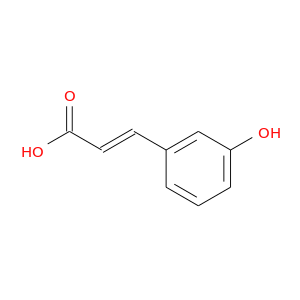
CID is 637541
synonyms found at PubChem are:
3-Hydroxycinnamic acid, 14755-02-3, 3-Coumaric acid, 588-30-7, M-COUMARIC ACID, m-Hydroxycinnamic acid, (E)-3-(3-Hydroxyphenyl)acrylic acid, trans-3-Hydroxycinnamic acid, 3-(3-Hydroxyphenyl)acrylic acid, trans-3-Hydroxycinnamate, Cinnamic acid, m-hydroxy-, (2E)-3-(3-hydroxyphenyl)prop-2-enoic acid, trans-3-coumaric acid, (2E)-3-(3-hydroxyphenyl)acrylic acid, (E)-3-(3-Hydroxyphenyl)-2-propenoic acid, UNII-KWJ2DDJ34H, 2-Propenoic acid, 3-(3-hydroxyphenyl)-, 2-propenoic acid, 3-(3-hydroxyphenyl)-, (2E)-, trans-m-Coumaric Acid, (E)-3-(3-hydroxyphenyl)prop-2-enoic acid, (2E)-3-(3-Hydroxyphenyl)-2-propenoic acid, NSC 28956, NSC 50308, KWJ2DDJ34H, 3-(3-hydroxyphenyl)-2-propenoic acid, CHEMBL98521, 3-(3-Hydroxyphenyl)acrylsaeure, CHEBI:32357, KKSDGJDHHZEWEP-SNAWJCMRSA-N, 2-Propenoic acid, 3-(3-hydroxyphenyl)-, (E)-, 3-hydroxycinnamate, trans-3-coumarate, 3-(3-hydroxyphenyl)prop-2-enoic acid, (2E)-3-(3-hydroxyphenyl)prop-2-enoate, 3-(3-hydroxyphenyl)acrylate, 3-(3-hydroxyphenyl)prop-2-enoate, (2E)-3-(3-hydroxyphenyl)acrylate, (E)-3-(3-hydroxyphenyl)-2-propenoate, m-Coumarate, 3-(3-Hydroxy-phenyl)-acrylic acid, m-Hydroxycinnamate, m-hydroxy-Cinnamate, EINECS 209-615-0, 3'-Hydroxycinnamate, trans-m-Cumaric Acid, PubChem8222, AC1LCUFW, m-hydroxy-Cinnamic acid, 3'-Hydroxycinnamic acid, 3-Hydroxy cinnamic acid, AI3-32389, 3/'-Hydroxycinnamic acid, bmse000093, 3-Hydroxyphenylacrylic acid, Cinnamic acid, 3-hydroxy-, SCHEMBL442408, Jsp002754, CHEBI:47925, MolPort-000-886-288, MolPort-023-134-755, ZINC155996, 3-(3-hydroxyphenyl)-2-Propenoate, ACT02256, ALBB-006261, NSC28956, NSC50308, m-Coumaric acid, analytical standard, trans-3-Hydroxycinnamic acid, 99%, BBL013143, BDBM50146456, MFCD00004390, NSC-28956, NSC-50308, SBB057740, STK400397, AKOS000146568, 2-Propenoicacid,3-(3-hydroxyphenyl)-, 3-(3-hydroxyphenyl)-(2E)-propenoate, RP17400, RTR-005890, (E)-3-(3-hydroxyphenyl)-acrylic acid, (E)-3-(3-hydroxy-phenyl)-acrylic acid, (2E)-3-(3-hydroxyphenyl)-2-propenoate, AC-16619, AJ-13662, AN-46147, AS-12450, BC210626, KB-02461, M680, SC-80962, ST097477, 3-(3-Hydroxyphenyl)-(2E)-propenoic acid, AB0011960, AB1003732, HY-113357, KB-183004, TC-169569, TR-005890, BB 0256480, CS-0062284, ST24028291, B-7286, C12621, J10101, M-2958, 2-Propenoic acid, 3-(3-hydroxyphenyl)- (9CI), 2-Propenoic acid,3-(3-hydroxyphenyl)-, (2E)-, A832036, I01-6597, J-501891, 358818FD-674F-4656-96AF-40F17C30F2EE, InChI=1/C9H8O3/c10-8-3-1-2-7(6-8)4-5-9(11)12/h1-6,10H,(H,11,12)/b5-4
ID: 18
InChIKey: REFJWTPEDVJJIY-UHFFFAOYSA-N SMILES: C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
biological descriptors:
CFTR relevance: CFTR activatorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
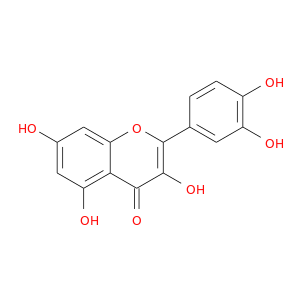
CID is 5280343
synonyms found at PubChem are:
quercetin, 117-39-5, Meletin, Sophoretin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, 3,3',4',5,7-Pentahydroxyflavone, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, Flavin meletin, Cyanidelonon 1522, T-Gelb bzw. grun 1, 3,5,7,3',4'-Pentahydroxyflavone, C.I. Natural Yellow 10, Quercetin content, Kvercetin, Quertin, C.I. Natural red 1, Kvercetin [Czech], Natural Yellow 10, C.I. 75670, CI Natural Yellow 10, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, 3',4',5,7-Tetrahydroxyflavan-3-ol, 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, C.I. Natural yellow 10 & 13, CCRIS 1639, HSDB 3529, Flavone, 3,3',4',5,7-pentahydroxy-, NCI-C60106, UNII-9IKM0I5T1E, NSC 9219, 3',4',5,7-tetrahydroxyflavon-3-ol, 3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-on, Cyanidenolon 1522, CHEBI:16243, AI3-26018, NSC9219, CHEMBL50, EINECS 204-187-1, MixCom3_000183, BRN 0317313, C.I . natural yellow 10, 9IKM0I5T1E, CI 75670, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one, 2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one, REFJWTPEDVJJIY-UHFFFAOYSA-N, KUC104418N, KUC107684N, 3,3',4,5,7-Pentahydroxyflavone, LIM-5662, LNS-5662, NSC-9219, TNP00070, TNP00089, KSC-23-76, KSC-10-126, P0042, DSSTox_CID_1218, Q 0125, DSSTox_RID_76017, DSSTox_GSID_21218, 74893-81-5, QUE, CU-01000012502-3, Q-200333, BRD9794, 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one, BRD-9794, CAS-117-39-5, NSC57655, NSC58588, SR-01000076098, Ritacetin, Quer, 4dfu, 4mra, Quercetin_sathishkumar, Quercetin (Sophoretin), Quercetin - Sophoretin, Spectrum_000124, Tocris-1125, 3cf8, AC1NQWX8, BiomolKI_000062, 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, zirconium(2+) salt (1:1), Maybridge1_008992, Prestwick0_000507, Prestwick1_000507, Prestwick2_000507, Prestwick3_000507, Spectrum2_000059, Spectrum3_000642, Spectrum4_000807, Spectrum5_001389, Lopac-Q-0125, C.I. natural yellow 13, BiomolKI2_000068, D0K8KX, Enicostemma Littorale Blume, UPCMLD-DP081, NCIOpen2_007628, NCIOpen2_007882, BIDD:PXR0007, Lopac0_000999, SCHEMBL19723, BSPBio_000433, BSPBio_001068, BSPBio_002243, KBioGR_000408, KBioGR_001293, KBioSS_000408, KBioSS_000584, 5-18-05-00494 (Beilstein Handbook Reference), KSC497C4F, MLS006011766, BIDD:ER0315, DivK1c_000485, SCHEMBL219729, SPECTRUM1500672, SPBio_000217, SPBio_002354, AC1Q795S, AC1Q795T, BDBM7460, BPBio1_000477, GTPL5346, MEGxp0_000381, SGCUT00001, 3,4',5,7-Pentahydroxyflavone, DTXSID4021218, UPCMLD-DP081:001, ACon1_000560, CTK3J7142, HMS501I07, KBio1_000485, KBio2_000408, KBio2_000584, KBio2_002976, KBio2_003152, KBio2_005544, KBio2_005720, KBio3_000775, KBio3_000776, KBio3_001463, 3,7,3',4'-Pentahydroxyflavone, MolPort-001-740-557, NINDS_000485, 3',5,7-Tetrahydroxyflavan-3-ol, Bio1_000369, Bio1_000858, Bio1_001347, Bio2_000374, Bio2_000854, HMS1362F09, HMS1792F09, HMS1923O19, HMS1990F09, HMS3263G19, HMS3267M12, HMS3649D04, HMS3656C15, to_000078, ZINC3869685, 3,5,7,3',4'-Pentahydroxyflavon, Tox21_202308, Tox21_300285, Tox21_500999, ANW-73134, BBL005513, BS0155, CCG-40054, CQ0011, Flavone,3',4',5,7-pentahydroxy-, GP9232, LMPK12110004, LS-589, MFCD00006828, NSC324608, Quercetin solution, 20 mM in DMSO, s2391, SBB012521, STK365650, Quercetin, >=95% (HPLC), solid, 3,4',5,5',7-pentahydroxy-Flavone, AKOS000511724, CS-3981, DB04216, DS-3416, EBD2197934, LP00999, MCULE-2433372790, NUT0000107, RTX-012622, IDI1_000485, IDI1_002129, KS-0000021G, SMP1_000252, Flavone, 3,4',5,5',7-pentahydroxy-, NCGC00015870-01, NCGC00015870-02, NCGC00015870-03, NCGC00015870-05, NCGC00015870-06, NCGC00015870-07, NCGC00015870-08, NCGC00015870-09, NCGC00015870-10, NCGC00015870-11, NCGC00015870-12, NCGC00015870-13, NCGC00015870-14, NCGC00015870-15, NCGC00015870-16, NCGC00015870-17, NCGC00015870-18, NCGC00015870-19, NCGC00015870-21, NCGC00015870-22, NCGC00015870-23, NCGC00015870-24, NCGC00025016-01, NCGC00025016-02, NCGC00025016-03, NCGC00025016-04, NCGC00025016-05, NCGC00025016-06, NCGC00025016-07, NCGC00025016-08, NCGC00168962-01, NCGC00168962-02, NCGC00168962-03, NCGC00168962-04, NCGC00254218-01, NCGC00259857-01, NCGC00261684-01, 4CN-0923, AC-19596, AC-29756, AJ-46321, AK106169, AN-22768, BAS 00649429, CJ-10980, HY-18085, KB-66753, LS-69030, NCI60_042036, S295, SC-25667, SMR000112559, ST024706, ST057237, Quercetin, Sophoretin, Meletin, Quercetine, AX8030401, KB-221421, EU-0100999, FT-0603318, FT-0655108, N1841, Q0025, ST24039236, Quercetin, disposable screening library format, Quercetin; 3,3',4',5,7-Pentahydroxyflavone, 17Q395, A-8821, C00389, K00029, S00057, WLN: T66 BO EVJ CR CQ DQ & DQ GQ IQ, SR-01000076098-1, SR-01000076098-3, SR-01000076098-7, SR-01000076098-8, BRD-K97399794-001-02-1, BRD-K97399794-001-07-0, BRD-K97399794-001-09-6, BRD-K97399794-001-11-2, BRD-K97399794-335-03-1, SR-01000076098-11, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate, 3,3',4',5,7-pentahydroxyflavone solution, 20 mM in DMSO, A1784/0075599, 49643640-FD4C-4B93-BD28-0D7C2021CC52, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one #, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one solution, 20 mM in DMSO, 73123-10-1
ID: 24
InChIKey: ZQSIJRDFPHDXIC-UHFFFAOYSA-N SMILES: C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3)O)O
biological descriptors:
CFTR relevance: medium CFTR correctorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
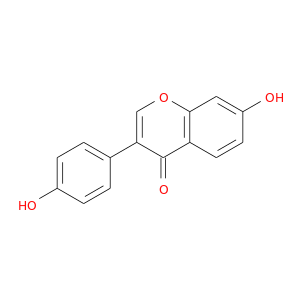
CID is 5281708
synonyms found at PubChem are:
daidzein, 486-66-8, 4',7-Dihydroxyisoflavone, Daidzeol, 7,4'-Dihydroxyisoflavone, 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one, Diadzein, 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-, 7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one, 7-Hydroxy-3-(4-hydroxyphenyl)-4-benzopyrone, UNII-6287WC5J2L, CCRIS 7600, K 251b, 4,7-Dihydroxyisoflavone, EINECS 207-635-4, BRN 0231523, CHEMBL8145, 4',7-Dihydroxy-iso-flavone, d-(+)-alpha-methylbenzylamine, 4′,7-Dihydroxyisoflavone, CHEBI:28197, ZQSIJRDFPHDXIC-UHFFFAOYSA-N, 6287WC5J2L, Daidzein,(S), 7-Hydroxy-3-(4-hydroxy-phenyl)-chromone, DSSTox_CID_2310, DSSTox_RID_76543, DSSTox_GSID_22310, Q-200924, CAS-486-66-8, SMR000326832, Daidzein (6CI), SR-01000075492, NPI 031E, PubChem9841, AC1NQYXY, Spectrum_000255, Tocris-1417, BiomolKI_000060, Spectrum2_000053, Spectrum3_000191, Spectrum4_001964, Spectrum5_000857, Lopac-D-7802, 4 ,7-Dihydroxyisoflavone, BiomolKI2_000066, D0N9CG, D0SY2M, UPCMLD-DP052, 4',7-dihydroxy isoflavone, 4',7-dihydroxy-Isoflavone, Lopac0_000412, Oprea1_182317, Oprea1_305345, SCHEMBL19814, BSPBio_001741, Daidzein, analytical standard, KBioGR_002432, KBioSS_000735, SPECTRUM200789, 5-18-04-00089 (Beilstein Handbook Reference), MLS000859973, MLS001304056, MLS006011853, BIDD:ER0120, DivK1c_001023, SPBio_000205, Daidzein, >=98%, synthetic, Isoflavone, 4',7-dihydroxy-, BIMB2005, BMK1-F12, GTPL2828, MEGxm0_000123, NPI-031E, d-(+)-alpha-methylbenzyl amine, DTXSID9022310, UPCMLD-DP052:001, ACon0_001477, ACon1_000543, BDBM23420, HMS503M07, K-251b, KBio1_001023, KBio2_000735, KBio2_003303, KBio2_005871, KBio3_001241, KS-00000MGD, AOB5524, OR1035T, 4',7-Dihydroxyisoflavone, 97%, MolPort-000-003-017, NINDS_001023, ZX-AFC000144, 7,4'-Dihydroxy-isoflavone (3a), HMS1922P18, HMS2233H24, HMS3261C06, HMS3267J04, HMS3370C03, HMS3468L18, HMS3649B20, HMS3655A18, ALBB-015933, BCP28286, HY-N0019, ZX-AN014640, ZX-AT022124, Tox21_201444, Tox21_303650, Tox21_500412, BBL010490, BS0244, CCG-38357, CD-190, LMPK12050038, MFCD00016954, s1849, SBB066056, STK801626, ZINC18847034, Daidzein (4',7-Dihydroxyisoflavone), 7-hydroxy-3-(4-hydroxyphenyl)-4H-, AKOS002385052, Isoflavone, 4',7-dihydroxy- (8CI), AC-6035, ACN-034764, CS-2332, Daidzein, purum, >=98.0% (TLC), KS-5260, LP00412, LS-2143, MCULE-8239511422, SDCCGMLS-0066422.P001, IDI1_001023, SMP1_000089, NCGC00015365-01, NCGC00015365-02, NCGC00015365-03, NCGC00015365-04, NCGC00015365-05, NCGC00015365-06, NCGC00015365-07, NCGC00015365-08, NCGC00015365-09, NCGC00015365-10, NCGC00015365-11, NCGC00015365-12, NCGC00015365-13, NCGC00015365-14, NCGC00015365-15, NCGC00015365-16, NCGC00015365-17, NCGC00015365-18, NCGC00025156-01, NCGC00025156-02, NCGC00025156-03, NCGC00025156-04, NCGC00025156-05, NCGC00025156-06, NCGC00025156-07, NCGC00025156-08, NCGC00025156-09, NCGC00025156-10, NCGC00168978-01, NCGC00168978-02, NCGC00257367-01, NCGC00258995-01, NCGC00261097-01, AJ-70673, AK-94232, AN-45238, CC-17424, KB-49550, SC-46504, ST057515, AB0010476, AB1004493, BB 0259520, D2668, EU-0100412, FT-0080019, FT-0603419, FT-0665448, N1862, R1341, 7-hydroxy-3-(4-hydroxyphenyl)-chromen-4-one, 7-Hydroxy-3-(4-hydroxy-phenyl)-chromen-4-one, 7-HYDROXY-3-(4-HYDROXYPHENYL)CHROMONE, C10208, D 7802, D17500, S00273, US8552057, 7, AB00052712-08, AB00052712_10, 486D668, C-15745, Daidzein, primary pharmaceutical reference standard, I06-0011, SR-01000075492-1, SR-01000075492-3, SR-01000075492-7, SR-01000075492-8, BRD-K42095107-001-02-3, BRD-K42095107-001-05-6, BRD-K42095107-001-08-0, SR-01000075492-12, 80E3ED75-D852-4D97-9BD6-B5ADE7EA25A1, 7-Hydroxy-3-(4-hydroxy-phenyl)-4H-1-benzo-pyran-4-one, UNII-71B37NR06D component ZQSIJRDFPHDXIC-UHFFFAOYSA-N, Daidzein, United States Pharmacopeia (USP) Reference Standard, Daidzein, Pharmaceutical Secondary Standard; Certified Reference Material, 4 inverted exclamation marka,7-Dihydroxyisoflavone; 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 7-Hydroxy-3-(4-hydroxyphenyl)chromone
ID: 645
InChIKey: FTVWIRXFELQLPI-ZDUSSCGKSA-N SMILES: C1[C@H](OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
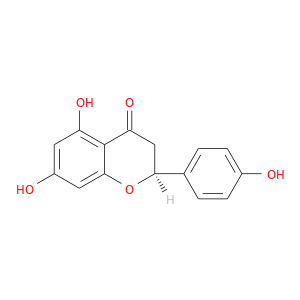
CID is 439246
synonyms found at PubChem are:
naringenin, 480-41-1, (S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one, (2S)-Naringenin, naringetol, pelargidanon, salipurpol, Asahina, (S)-Naringenin, Salipurol, UNII-HN5425SBF2, CCRIS 5839, C15H12O5, YSO1, (-)-(2S)-Naringenin, CHEMBL9352, (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, AI3-23355, HN5425SBF2, CHEBI:17846, (-)-Naringenin, Flavanone, 4',5,7-trihydroxy- (8CI), NSC 11855, (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one, 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (2S)-, NAR, EINECS 207-550-2, pelargidanon 1602, (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, SR-01000721771, (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one, (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone, NSC-11855, 2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, 2uxu, 4deu, Spectrum_000247, 4eh3, Spectrum2_000325, Spectrum3_000567, Spectrum4_000124, Spectrum5_001423, D02ABO, 4',5,7-triOH-Flavone, AC1L96YW, SCHEMBL20570, BSPBio_001954, KBioGR_000508, KBioSS_000727, MLS000574861, BIDD:ER0116, DivK1c_000118, SPECTRUM1500746, SPBio_000329, 4',5, 7-Trihydroxyflavanone, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, AC1Q78P1, DTXSID1022392, BDBM23419, CTK8C4789, HMS500F20, KBio1_000118, KBio2_000727, KBio2_003295, KBio2_005863, KBio3_001454, FTVWIRXFELQLPI-ZDUSSCGKSA-N, MolPort-001-796-145, NINDS_000118, (2S)-5,7,4'-trihydroxyflavone, AIDS001417, HMS2202M06, ZINC156701, (2S)-4',5,7-trihydroxyflavanone, HY-N0100, KS-000010IV, TNP00287, 2580AH, ANW-73132, CCG-38601, LMPK12140001, Phytochemistry 8: 127 (1969), s2394, AKOS016843490, CS-6421, DB03467, RTR-017609, SDCCGMLS-0066570.P001, (2S)-4',5,7-trihydroxyflavan-4-one, IDI1_000118, NCGC00016457-01, NCGC00016457-02, NCGC00016457-03, NCGC00017346-01, NCGC00163598-01, AJ-13871, CAS-480-41-1, SMR000156272, ZB006427, FT-0617134, FT-0617135, C00509, Q-100666, SR-01000721771-3, SR-01000721771-4, BRD-K08832567-001-02-4, BRD-K08832567-001-06-5, (-)-(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one, (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one, (S)-2,3-dihydo-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, 13308-00-4, 15912-71-7
ID: 105
InChIKey: ADFCQWZHKCXPAJ-LBPRGKRZSA-N SMILES: C1[C@@H](COC2=C1C=CC(=C2)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: potent ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
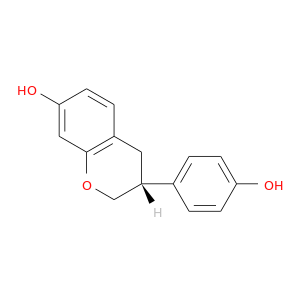
CID is 6950272
synonyms found at PubChem are:
(R)-Equol, R-Equol, Equol, 221054-79-1, UNII-2V8RAP1HXA, 2V8RAP1HXA, Equol, (+)-, (R)-3-(4-hydroxyphenyl)chroman-7-ol, (3R)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol, Isoequol, (+)-Equol, AC1OCT9N, SCHEMBL2002128, CHEMBL3127843, MolPort-002-507-289, ZINC388660, EX-A1353, KS-000010MK, 1091AH, BBL026747, STK801893, AKOS004120071, CS-8182, MCULE-6555642673, AJ-20721, CJ-03316, ZB011639, HY-108414, FT-0772959, UNII-2RZ8A7D0E8 component ADFCQWZHKCXPAJ-LBPRGKRZSA-N, 2H-1-Benzopyran-7-ol, 3,4-dihydro-3-(4-hydroxyphenyl)-, (3R)-
ID: 16
InChIKey: JWZZKOKVBUJMES-UHFFFAOYSA-N SMILES: CC(C)NCC(C1=CC(=C(C=C1)O)O)O
biological descriptors:
CFTR relevance: stimulates Cl- conductance as agonist of β2 adrenergic receptorCategory:
Influence on CFTR function enhances CFTR function
Order of interaction indirect
subcellular compartment Apical membrane & subapical compartment
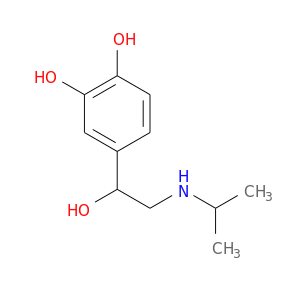
CID is 3779
synonyms found at PubChem are:
isoproterenol, Isoprenaline, Isoprenalin, Norisodrine, Novodrin, Isopropydrin, Isopropylarterenol, Asiprenol, Assiprenol, Bellasthman, Respifral, Aludrin, Aludrine, Asmalar, Isadrine, N-Isopropylnoradrenaline, Bronkephrine, Isonorene, Isopropyladrenaline, Lomupren, Neodrenal, Proternol, N-Isopropylnorepinephrine, Isopropylnorepinephrine, 7683-59-2, neo-Epinine, Isonorin, Isorenin, Saventrine, Isopropylnoradrenaline, Isopropyl noradrenaline, Racemic isoprenaline, dl-Isadrine, Isuprel, Racemic isoproterenol, (+-)-Isoproterenol, Vapo-N-iso, Epinephrine isopropyl homolog, Isoprenalinum, Isoproterenolum, Aleudrine, Isupren, (+-)-Isoprenaline, dl-Ipr, Isoprenalina, Isadrin, Isoproterenol [JAN], Dihydroxyphenylethanolisopropylamine, WIN 5162, DL(+-)-Isoproterenol, Medihaler-ISO, dl-Isopropylnoradrenaline, DL-Isopropylnorepinephrine, dl-N-Isopropylnoradrenaline, Isoprenalinum [INN-Latin], Isoprenalina [INN-Spanish], Isoproterenol Chloride, 1-(3,4-Dihydroxyphenyl)-2-(isopropylamino)ethanol, ICI 46399, l-Isoproterenol, d-Isoprenaline, d-Isoproterenol, alpha-(Isopropylaminomethyl)protocatechuyl alcohol, Isuprel Mistometer, N-Isopropyl-beta-dihydroxyphenyl-beta-hydroxyethylamine, (S)-Isoproterenol, (+)-Isoprenaline, d-Isopropylarterenol, Aleudrin, 1-(3,4-Dihydroxyphenyl)-2-isopropylaminoethanol, Isoprenaline (INN), ISOPROP, CCRIS 3081, 4-(1-Hydroxy-2-((1-methylethyl)amino)ethyl)-1,2-benzenediol, l-Isopropylnoradrenaline, NSC 9975, EINECS 231-687-7, NSC 33791, (S)-(+)-Isoproterenol, Isoproterenol sulfate, Isopropylaminomethyl-3,4-dihydroxyphenyl carbinol, CHEMBL434, d-N-Isopropylnorepinephrine, 1,2-Benzenediol, 4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-, BRN 2213857, 3,4-Dihydroxy-alpha-((isopropylamino)methyl)benzyl alcohol, 4-[1-hydroxy-2-(propan-2-ylamino)ethyl]benzene-1,2-diol, CHEBI:64317, 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, JWZZKOKVBUJMES-UHFFFAOYSA-N, (S)-Isoprenaline, Isopropylaminomethyl(3,4-dihydroxyphenyl)carbinol, NSC33791, (+)-Isoproterenol, Isoprenaline [INN], Protocatechuyl alcohol, alpha-(isopropylaminomethyl)-, DL-ISOPROTERENOL, NCGC00015558-06, 3,4-Dihydroxy-.alpha.-(isopropylaminomethyl)-benzyl alcohol, Protocatechuyl alcohol,-, Isoprenaline sulfate, Isuprel (TN), DSSTox_CID_3175, 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, (+-)-, BENZYL ALCOHOL, 3,4-DIHYDROXY-alpha-((ISOPROPYLAMINO)METHYL)-, (+-)-, DSSTox_RID_76904, DSSTox_GSID_23175, 149-53-1, .alpha.-(Isopropylaminomoethyl)protocatechuyl alcohol, 4-(1-Hydroxy-2(isopropylamino)ethyl)-benzene 1,2-diol, 4-(1-Hydroxy-2-(isopropylamino)ethyl)benzene-1,2-diol, 3,4-dihydroxy-alpha-[(isopropylamino)methyl]benzyl alcohol, WLN: QR BQ DYQ1MY1&1, 3,4-Dihydroxy-.alpha.-[(isopropylamino)methyl]benzyl alcohol, 4-{1-hydroxy-2-[(1-methylethyl)amino]ethyl}benzene-1,2-diol, Benzyl alcohol, 3,4-dihydroxy-alpha-((isopropylamino)methyl)-, CAS-7683-59-2, 1, 4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-, 4-[1-hydroxy-2-(isopropylamino)ethyl]benzene-1,2-diol, 4-{1-hydroxy-2-[(methylethyl)amino]ethyl}benzene-1,2-diol, Benzyl alcohol,4-dihydroxy-.alpha.-[(isopropylamino)methyl]-, Isoproterenol-l, Isoproterenol;, Isoprenalinesulphate, Isoproterenol (-), Isoproterenol,(+), DL-isoprenaline HCl, ISOPROTERONOL, Isuprel (Salt/Mix), Izadrin (Salt/Mix), Euspiran (Salt/Mix), (+/-)-isoprenaline, (+/-)-isoproterenol, Spectrum_000949, AC1L1GOZ, AC1Q1QBS, 6700-39-6, Prestwick0_001097, Prestwick1_001097, Prestwick2_001097, Spectrum2_001061, Spectrum3_000474, Spectrum4_000024, Spectrum5_000880, (.+/-.)-Isoprenaline, (.+/-.)-Isoproterenol, D0I8FI, D0IF0C, D0P1IS, SCHEMBL4165, DL(.+/-.)-Isoproterenol, Lopac0_000711, Oprea1_009434, BSPBio_002208, GTPL536, KBioGR_000427, KBioSS_001429, 3-13-00-02387 (Beilstein Handbook Reference), DivK1c_000894, SPBio_001042, SPBio_003057, SGCUT00015, BENZYL ALCOHOL, 3,4-DIHYDROXY-alpha-((ISOPROPYLAMINO)METHYL)-, (+)-, DTXSID4023175, BDBM25392, KBio1_000894, KBio2_001429, KBio2_003997, KBio2_006565, KBio3_001428, NSC9975, NINDS_000894, HMS2089A12, BCP09043, NSC-9975, to_000062, (A+/-)-Isoproterenol hydrochloride, Tox21_110172, AS1409, NSC-33791, PDSP1_001425, PDSP2_001409, SBB005855, AKOS015913894, Tox21_110172_1, API0006523, CCG-204727, CCG-204796, DB01064, MCULE-6061231962, IDI1_000894, NCGC00015558-04, NCGC00015558-05, NCGC00015558-07, NCGC00015558-08, NCGC00015558-09, NCGC00015558-10, NCGC00015558-11, NCGC00015558-12, NCGC00015558-14, NCGC00016665-02, NCGC00025274-03, NCGC00025274-04, NCGC00162220-01, LS-42866, LS-42868, LS-42869, ST077771, SBI-0050689.P004, FT-0724367, C07056, D08090, EN300-148243, AB00053487-09, AB00053487-10, AB00053487_11, AB00053487_12, 683I592, alpha-(Isopropylaminomoethyl)protocatechuyl alcohol, L000936, .alpha.-(Isopropylaminomethyl)protocatechuyl alcohol, I14-45263, Protocatechuyl alcohol,.alpha.-(isopropylamino-methyl),-, 3,4-dihydroxy-alpha-[(isopropylamino)methyl]-benzyl alcohol, 4-[1-Hydroxy-2-(isopropylamino)ethyl]-1,2-benzenediol #, N-Isopropyl-.beta.-dihydroxyphenyl-.beta.-hydroxyethylamine, 4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;hydrochloride, 4-{1-hydroxy-2-[(propan-2-yl)amino]ethyl}benzene-1,2-diol, 4-{1-hydroxy-2-[(propan-2-yl)amino]ethyl}benzene-1,2-diol, 2, Benzyl alcohol, 3,4-dihydroxy-.alpha.-((isopropylamino)methyl)-, 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)- (9CI), 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-(9CI), 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, (S)-, 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, (S)-(9CI), 46388-38-9
ID: 85
InChIKey: ZZIALNLLNHEQPJ-UHFFFAOYSA-N SMILES: C1=CC2=C(C=C1O)OC3=C2C(=O)OC4=C3C=CC(=C4)O
biological descriptors:
CFTR relevance: ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
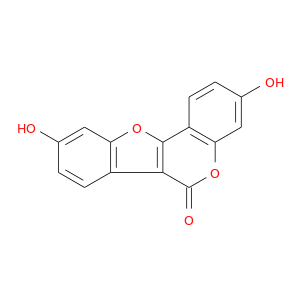
CID is 5281707
synonyms found at PubChem are:
COUMESTROL, 479-13-0, Cumoestrol, Cumoesterol, Cumostrol, Coumesterol, 3,9-Dihydroxy-6H-[1]benzofuro[3,2-c]chromen-6-one, 7,12-Dihydroxycoumestan, Cumestrol, 3,9-Dihydroxycoumestan, UNII-V7NW98OB34, NSC 22842, CCRIS 7311, NSC22842, 6H-Benzofuro[3,2-c][1]benzopyran-6-one, 3,9-dihydroxy-, EINECS 207-525-6, BRN 0266702, CHEMBL30707, MLS000738006, CHEBI:3908, V7NW98OB34, ZZIALNLLNHEQPJ-UHFFFAOYSA-N, 3,9-Dihydroxy-6H-benzofuro(3,2-c)(1)benzopyran-6-one, 6H-Benzofuro(3,2-c)(1)benzopyran-6-one, 3,9-dihydroxy-, 3,9-dihydroxybenzofuro[3,2-c]chromen-6-one, A1-00298, SMR000059001, AC1NQYXV, DSSTox_CID_2399, D02DML, NCIMech_000078, DSSTox_RID_76572, DSSTox_GSID_22399, Oprea1_222511, SCHEMBL22012, 5-19-06-00405 (Beilstein Handbook Reference), MLS000069446, BIDD:ER0114, ZINC1219, DTXSID6022399, BDBM23451, CTK8F8799, MolPort-003-846-031, HMS2235B05, HMS3374A07, 6H-Benzofuro[3, 3,9-dihydroxy-, Coumestrol, >=95.0% (HPLC), HY-N2335, Tox21_200032, CCG-35536, CCG-36200, LMPK12090018, MFCD00016885, NSC-22842, AKOS028111776, AN-6463, API0002121, CS-6343, VZ31590, 3-Benzofurancarboxylic acid, 2-(2,4-dihydroxyphenyl)-6-hydroxy-, delta-lactone (6CI), SMP2_000163, NCGC00018124-01, NCGC00018124-02, NCGC00018124-03, NCGC00018124-04, NCGC00018124-05, NCGC00018124-06, NCGC00023462-03, NCGC00023462-04, NCGC00257586-01, 4CN-2508, CAS-479-13-0, CC-25990, LS-35394, NCI60_001863, ZB000278, FT-0603177, ST50320052, V0359, C10205, S00280, US8552057, 3, 3,9-dihydroxy-[1]benzofuro[3,2-c]chromen-6-one, 3,9-dihydroxy-[1]benzoxolo[3,2-c]chromen-6-one, 3,9-dihydroxy-6H-benzofuro[3,2-c]chromen-6-one, 3,9-dihydroxybenzo[d]chromeno[4,3-b]furan-6-one, A827386, C-16836, 3,9-Dihydroxy-benzo[4,5]furo[3,2-c]chromen-6-one, BRD-K97509413-001-01-8, 3,9-bis(oxidanyl)-[1]benzofuro[3,2-c]chromen-6-one, 3,9-Dihydroxy-6H-[1]benzofuro[3,2-c]chromen-6-one #, 3,9-Dihydroxy-6H-benzofuro[3,2-c]-[1]benzopyran-6-one, 6H-Benzofuro(3,2-c)(1)benzopyran-6-one, 3,9-dihdyroxy-, 6H-Benzofuro[3,2-c][1]benzopyran-6-one,3,9-dihydroxy-, Coumestrol, BioReagent, suitable for fluorescence, >=97.5% (HPLC), 2-(2,4-Dihydroxyphenyl)-6-hydroxy-3-benzofurancarboxylic Acid |A-Lactone, 3-Benzofurancarboxylic acid,4-dihydroxyphenyl)-6-hydroxy-, .delta.-lactone, 3-Benzofurancarboxylic acid, 2-(2,4-dihydroxyphenyl)-6-hydroxy-, .delta.-lactone, 3-Benzofurancarboxylic acid, 2-(2,4-dihydroxyphenyl)-6-hydroxy-, delta-lactone, 5,14-dihydroxy-8,17-dioxatetracyclo[8.7.0.0^{2,7}.0^{11,16}]heptadeca-1(10),2,4,6,11(16),12,14-heptaen-9-one
ID: 106
InChIKey: ADFCQWZHKCXPAJ-UHFFFAOYSA-N SMILES: C1C(COC2=C1C=CC(=C2)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: potent ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
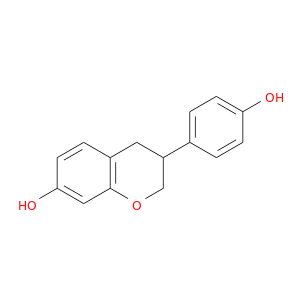
CID is 382975
synonyms found at PubChem are:
94105-90-5, 3-(4-hydroxyphenyl)chroman-7-ol, Equol, (+/-)-Equol, (R,S)-Equol, 7,4'-Homoisoflavane, 4',7-Dihydroxyisoflavane, (RS)-Equol, 3-(4-hydroxyphenyl)-7-chromanol, ( inverted exclamation markA)-Equol, 2H-1-Benzopyran-7-ol, 3,4-dihydro-3-(4-hydroxyphenyl)-, 4',7-Isoflavandiol, ADFCQWZHKCXPAJ-UHFFFAOYSA-N, 3-(4-Hydroxy-phenyl)-chroman-7-ol, 3,4-Dihydro-3-(4-hydroxyphenyl)-2H-1-benzopyran-7-ol, 3-(4-Hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol, 66036-38-2, 4',7-Dihydroxyisoflavan, 3-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol, 7,4'-dihydroxyisoflavan, Equol, (+/-)-, Rac-Equol, R,S-Equol, (+/-)Equol, AC1L8KFS, 4',7-Dihydroxyflavandiol, AC1Q7A6Q, (.+/-.)-Equol, SCHEMBL43648, SCHEMBL949461, BIE531, CHEMBL1957037, DTXSID7058705, 531-95-3 (S-isomer), CTK5H4974, NV 07|A, EQUOL,(+/-)-(SH), MolPort-003-847-077, ZX-AFC000526, HMS3648G10, HMS3651B05, EX-A1352, KS-00000Q4S, ZX-AT019275, (+/-) 7,4'-Dihydroxy Isoflavane, ANW-43844, GP9597, LMPK12080003, MFCD00016662, NSC670882, 7-Hydroxy-3-(4-hydroxyphenyl)chroman, AKOS015899541, CS-6870, DS-2344, HY-100583A, MCULE-1358694468, NSC-670882, OR60026, VA10827, SMP2_000187, AK-99885, KB-50551, SC-94870, (S)-3-(4-Hydroxy-phenyl)-chroman-7-ol, AX8232306, RT-012437, (+/-)-Equol, >=99.0% (TLC), 4 inverted exclamation mark ,7-Isoflavandiol, 4CH-011868, E0922, FT-0081331, FT-0660903, ST24022579, ST50331725, W6768, Z5370, M-9676, SR-01000946366, I06-0305, J-014504, SR-01000946366-1, BRD-A74907996-001-01-8, I14-11532, (+/-)-3,4-Dihydro-3-(4-hydroxyphenyl)-2H-chromen-7-ol, 2H-1-Benzopyran-7-ol,3,4-dihydro-3-(4-hydroxyphenyl)-
ID: 1070
InChIKey: JHYXBPPMXZIHKG-UHFFFAOYSA-N SMILES: C1C(C(=O)C2=C(O1)C=C(C=C2)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: unspecifiedCategory:
Influence on CFTR function unknown
Order of interaction unknown
subcellular compartment unknown
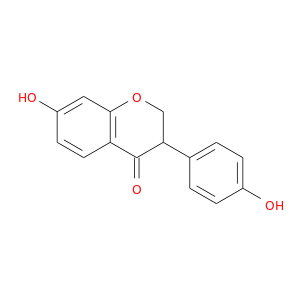
CID is 176907
synonyms found at PubChem are:
dihydrodaidzein, 17238-05-0, 7-hydroxy-3-(4-hydroxyphenyl)chroman-4-one, Dihydrodaidzein (keto), Dihydrodazein, (+/-)-Dihydrodaidzein, Dihydro Daidzein, 7,4'-Dihydroxyisoflavanone, 4',7-Dihydroxyisoflavanone, JHYXBPPMXZIHKG-UHFFFAOYSA-N, 2,3-dihydro-7-hydroxy-3-(4-hydroxyphenyl)-4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, Isoflavanone, 4',7-dihydroxy-, AC1L42FE, 4',7-dihydroxy-Isoflavanone, (R,S)-2,3-Dihydrodaidzein, SCHEMBL131543, SCHEMBL16152440, CHEBI:75842, CTK8C3008, BID0517, MolPort-003-846-731, ZX-AFC001619, HY-N1461, ANW-69504, IN2311, LMPK12050447, AKOS016006054, ACM17238050, AK-61048, KB-82949, AX8208356, LS-193361, TC-158249, 7-Hydroxy-3-(4-hydroxyphenyl)-4-chromanone, CS-0016908, D4239, FT-0666948, J-010813, 7-hydroxy-3-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one
ID: 35
InChIKey: FTVWIRXFELQLPI-UHFFFAOYSA-N SMILES: C1C(OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O
biological descriptors:
CFTR relevance: ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
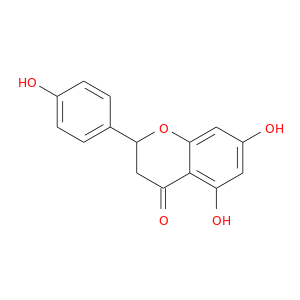
CID is 932
synonyms found at PubChem are:
naringenin, 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one, 67604-48-2, 4',5,7-Trihydroxyflavanone, Naringenine, (+/-)-Naringenin, 480-41-1, naringetol, salipurpol, (-)-Naringenin, NARIGENIN, Salipurol, 93602-28-9, 5,7,4'-Trihydroxyflavanone, (S)-Naringenin, NSC 34875, ( inverted exclamation markA)-Naringenin, CHEMBL32571, MLS000028739, MLS000738094, CHEBI:50202, Flavanone, 4',5,7-trihydroxy-, FTVWIRXFELQLPI-UHFFFAOYSA-N, NSC11855, NSC34875, SMR000059039, 4',7-Trihydroxyflavanone, NSC 11855, Flavanone,5,7-trihydroxy-, Q-100521, EINECS 207-550-2, 5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one, pelargidanon 1602, 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (S)-, naringenin-7-sulfate, NSC-34875, Narngenn, rac Naringenin, CCRIS 8135, (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone, NSC-11855, 4H-1-Benzopyran-4-one,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, 2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, 4H-1-Benzopyran-4-one,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (S)-, Naringenin (NAR), Prestwick_531, EINECS 266-769-1, Naringenin, 90%, (R,S)-Naringenin, BE 14348A, BE-14348A, Opera_ID_106, AC1L1ACN, AC1Q6KJD, Prestwick0_000466, Prestwick1_000466, Prestwick2_000466, Prestwick3_000466, Oprea1_194140, SCHEMBL20571, 4 ,5,7-Trihydroxyflavanone, BSPBio_000572, MLS001146907, SPBio_002511, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, BPBio1_000630, MEGxp0_000358, SCHEMBL1934259, ACon1_000582, BDBM19461, CTK7J9188, DTXSID50274239, MolPort-000-861-091, (+/-)-Naringenin, >=95%, HMS1569M14, HMS2096M14, HMS2231O18, HMS3352B08, HMS3373N07, HMS3656G15, Naringenin, natural (US), 98%, KS-000001ZW, ZX-AT026530, ANW-43843, BN0800, CN0031, SBB006461, Naringenin solution, 20 mM in DMSO, (+/-)-5,7,4'-Trihydroxyflavanone, 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, AKOS015895052, (+/-)-Naringenin, analytical standard, CS-W012357, KS-5142, LS-5089, MCULE-5489217450, RL05877, (+/-)-Naringenin, ~95% (HPCE), KS-00000R53, SMP1_000060, NCGC00017346-02, NCGC00017346-03, NCGC00017346-04, NCGC00095963-01, NCGC00095963-02, NCGC00095963-03, 4CN-0924, AC-20273, AK122638, AN-45184, K702, LS-39547, SC-18078, ST057236, AB1003941, AB1009825, AX8246816, KB-187979, KB-196356, ST2418556, TR-017609, TR-037462, 4CH-012369, FT-0082287, FT-0630981, N0072, M-7677, Naringenin, disposable screening library format, S00279, 5,7-Dihydroxy-2-(4-hydroxy-phenyl)-chroman-4-one, I06-0445, I06-0536, J-523457, 4',5,7-Trihydroxyflavanone solution, 20 mM in DMSO, BRD-A94669766-001-02-6, BRD-A94669766-001-04-2, 5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one, EC19096C-4404-4A16-9BF9-92F9F358E005, ( )-Naringenin; 4?,5,7-Trihydroxyflavanone; H-1-benzopyran-4-one, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, (S)- #, ( inverted exclamation markA)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4 inverted exclamation marka,5,7-Trihydroxyflavanone
ID: 43
InChIKey: JIMHYXZZCWVCMI-ZSOIEALJSA-N SMILES: C1=CC(=CC(=C1)N2C(=O)/C(=C/C3=CC=C(C=C3)C(=O)O)/SC2=S)C(F)(F)F
biological descriptors:
CFTR relevance: CFTR inhibitorCategory:
Influence on CFTR function inhibits CFTR function
Order of interaction unknown
subcellular compartment unknown
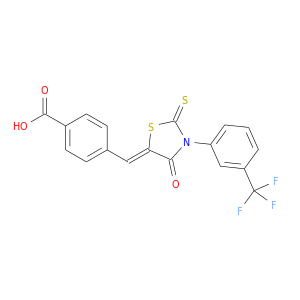
CID is 1554208
synonyms found at PubChem are:
CFTRinh-172, CFTR(inh)-172, CFTR Inhibitor-172, 307510-92-5, CHEMBL461939, 3-[(3-Trifluoromethyl)phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone, CFTRinh 172, 4-[4-oxo-2-thioxo-3-(3-trifluoromethyl-phenyl)-thiazolidin-(5z)-ylidenemethyl]-benzoic acid, CFinh-172, CFTR Inh-172, AC1LT95A, AOB6719, 5-[(4-Carboxyphenyl)methylene]-2-thioxo-3-[(3-trifluoromethyl)phenyl-4-thiazolidinone, JIMHYXZZCWVCMI-ZSOIEALJSA-N, MolPort-000-501-417, ZINC966859, BDBM50244767, MFCD06411408, STL032754, AKOS000365470, CS-3185, BAS 00786641, HY-16671, BIM-0044592.P001, ST50148666, EC-000.1908, Z-2135, J-018109, 4-((4-oxo-2-thioxo-3-(3-(trifluoromethyl)phenyl)thiazolidin-5-ylidene)methyl)benzoic acid, 4-[[4-Oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-5-thiazolidinylidene]methyl]benzoic acid, 4-[4-Oxo-2-thioxo-3-(3-trifluoromethyl-phenyl)-thiazolidin-5Z)-ylidenemethyl]-benzoic acid, (Z)-4-((4-oxo-2-thioxo-3-(3-(trifluoromethyl)phenyl)thiazolidin-5-ylidene)methyl)benzoic acid, 4-({4-oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene}met hyl)benzoic acid, 4-[(Z)-[4-oxo-2-sulfanylidene-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene]methyl]benzoic acid, 4-[(Z)-{4-oxo-2-thioxo-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene}methyl]benzoic acid, 4-{[(5Z)-4-oxo-2-sulfanylidene-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene]methyl}benzoic acid
ID: 1724
InChIKey: PMOWTIHVNWZYFI-AATRIKPKSA-N SMILES: C1=CC=C(C(=C1)/C=C/C(=O)O)O
biological descriptors:
CFTR relevance: unspecifiedCategory:
Influence on CFTR function unknown
Order of interaction unknown
subcellular compartment unknown
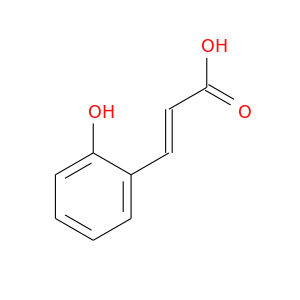
CID is 637540
synonyms found at PubChem are:
2-Hydroxycinnamic acid, o-Coumaric acid, 614-60-8, trans-2-Hydroxycinnamic acid, 2-Coumaric acid, trans-o-Hydroxycinnamic acid, trans-o-Coumaric acid, 2-Coumarate, (E)-o-Hydroxycinnamic acid, 2-Hydroxycinnamate, 583-17-5, o-Hydroxy-trans-cinnamic acid, trans-2-Hydroxycinnamate, (2E)-3-(2-hydroxyphenyl)prop-2-enoic acid, 3-(2-hydroxyphenyl)acrylic acid, (E)-3-(2-Hydroxyphenyl)-2-propenoic acid, (2E)-3-(2-HYDROXYPHENYL)ACRYLIC ACID, 2-Hydroxycinnamic acid, (E)-, CINNAMIC ACID, o-HYDROXY-, (E)-, UNII-23AU5FZB9C, (E)-2-hydroxycinnamic acid, CCRIS 5834, 2-Hydroxycinamic acid, trans-2-coumaric acid, (E)-3-(2-hydroxyphenyl)prop-2-enoic acid, (2E)-3-(2-Hydroxyphenyl)-2-propenoic acid, EINECS 210-386-4, ortho-Hydroxycinnamic acid, NSC 32952, (E)-3-(2-hydroxyphenyl)acrylic acid, 2-Propenoic acid, 3-(2-hydroxyphenyl)-, (E)-, 2-Propenoic acid, 3-(2-hydroxyphenyl)-, (2E)-, BRN 1100900, 23AU5FZB9C, 2-Propenoic acid, 3-(2-hydroxyphenyl)-, CHEMBL52564, CHEBI:18125, PMOWTIHVNWZYFI-AATRIKPKSA-N, 3-(2-hydroxyphenyl)prop-2-enoic acid, o-coumarate, 2-Hydroxy Cinnamic Acid, (E)-3-(2-HYDROXY-PHENYL)-ACRYLIC ACID, 2-HYDROXYCINNAMICACID, cis-2-coumaric acid, 2HC, cis-2-coumarate, 3-(2-hydroxyphenyl)prop-2-enoate, cis-2-hydroxycinnamic acid, o-Hydroxycinnamate, trans-o-Coumarate, (2Z)-3-(2-hydroxyphenyl)acrylic acid, ortho-coumaric acid, ortho-Hydroxycinnamate, PubChem8214, (E)-Coumarinic Acid, (2Z)-3-(2-hydroxyphenyl)acrylate, AC1LCUFT, trans-o-Hydroxycinnamate, trans-ortho-coumaric acid, (E)-ortho-coumaric acid, o-Hydroxy-trans-cinnamate, (E)-o-Hydroxycinnamicacid, bmse000347, Cinnamic acid, o-hydroxy-, WLN: QV1U1R BQ, AC1Q71FE, SCHEMBL64885, QSPL 150, CHEBI:18176, 2-Hydroxycinnamic acid, (2E)-, MolPort-001-641-053, ZINC895911, ALBB-025832, NSC32952, ZX-AT016055, BBL013048, BDBM50146462, MFCD00004379, NSC-32952, SBB065726, STK301745, AKOS003790794, 3-(2-Hydroxyphenyl)-2-propenoic acid, DB01650, MCULE-5451854573, OR40640, RTR-037283, (E)-3-(2-hydroxyphenyl)-acrylic acid, CINNAMIC ACID,2-HYDROXY (TRANS), trans-3-(2-hydroxyphenyl)propenoic acid, AJ-24216, AN-46543, AS-12449, KB-86220, LS-54110, ST097457, TR-037283, R1101, C01772, 2-Hydroxycinnamic acid, predominantly trans, 97%, 614H608, C-04438, I04-0024, 2-Propenoic acid, 3-(2-hydroxyphenyl)-, (E)- (9CI), F2191-0203, 90E8F55A-AB69-4720-95AF-747C2DCA5471, UNII-2S0H1PX3LM component PMOWTIHVNWZYFI-AATRIKPKSA-N
ID: 23
InChIKey: ZNNLBTZKUZBEKO-UHFFFAOYSA-N SMILES: COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3
biological descriptors:
CFTR relevance: CFTR BlockerCategory:
Influence on CFTR function inhibits CFTR function
Order of interaction binds to CFTR
subcellular compartment Apical membrane & subapical compartment
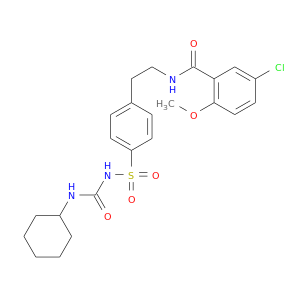
CID is 3488
synonyms found at PubChem are:
Glibenclamide, glyburide, 10238-21-8, Glybenclamide, Micronase, Diabeta, Glynase, Daonil, Euglucon, Maninil, Semi-daonil, Apo-Glibenclamide, Euglucon 5, Azuglucon, Bastiverit, Benclamin, Betanase, Duraglucon, Euglucan, Euglykon, Glibenil, Glucolon, Neogluconin, Orabetic, Prodiabet, Renabetic, Yuglucon, Dibelet, Gilemal, Glibens, Glibil, Glimel, Glimide, Humedia, Libanil, Suraben, Tiabet, Adiab, Glybenzcyclamide, Melix, Pira, Med-Glionil, Gewaglucon, Glibenbeta, Glibenclamida, Glibenclamidum, Glucohexal, Glucoremed, Hexaglucon, Lisaglucon, Normoglucon, Praeciglucon, Calabren, Cytagon, Euclamin, Glamide, Glibesyn, Glibetic, Gliboral, Glidiabet, Glisulin, Glitisol, Glubate, Glucobene, Glucomid, Glucoven, Glycomin, Lederglib, Miglucan, Debtan, Gliban, Gliben, Glibet, Glicem, Gluben, Glyben, Nadib, Sugril, Novo-Glyburide, Glibenclamid AL, Gen-Glybe, Norglicem 5, Betanese 5, Glibenclamid Fabra, Glibenclamid Basics, Glibenclamid-Cophar, Glibenclamid Heumann, Semi-Euglucon, Glibenclamid Genericon, Hemi-Daonil, Glibenclamid-Ratiopharm, Glibenclamidum [INN-Latin], Glibenclamida [INN-Spanish], Abbenclamide, HB 419, Diabiphage, Glibadone, Semi-Gliben-Puren N, GBN 5, Glibenclamid Riker M., Glyburide (micronized), Euglucon N, 5-Chloro-N-(4-(N-(cyclohexylcarbamoyl)sulfamoyl)phenethyl)-2-methoxybenzamide, Micronized glyburide, Glyburide [USAN], UR 606, Glimidstata, Glycolande, Glycron, PresTab, Gluco-Tablimen, Dia-basan, UNII-SX6K58TVWC, 1-(p-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesulfonyl)-3-cyclohexylurea, Gliben-Puren N, Micronase (TN), C23H28ClN3O5S, Glyburide (USP), HB-419, 1-((p-(2-(5-Chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-cyclohexylurea, Diabeta (TN), Glynase (TN), Glibenclamid Riker M, 5-Chloro-N-(2-(4-((((cyclohexylamino)carbonyl)amino)sulfonyl)phenyl)ethyl)-2-methoxybenzamide, U 26452, glyburide (glibenclamide), EINECS 233-570-6, HB-420, SX6K58TVWC, CHEMBL472, BRN 2230085, N-(4-(2-(5-Chloro-2-methoxybenzamido)ethyl)phenylsulfonyl)-N'-cyclohexylurea, MLS000069721, CHEBI:5441, 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide, Urea, 1-(p-(2-(5-chloro-2-methoxybenzamido)ethyl)benzenesulfonyl)-3-cyclohexyl-, Benzamide, 5-chloro-N-(2-(4-((((cyclohexylamino)carbonyl)amino)sulfonyl)phenyl)ethyl)-2-methoxy-, NCGC00015467-11, SMR000058229, CAS-10238-21-8, 5-Chloro-N-[4-(cyclohexylureidosulfonyl)phenethyl]-2-methoxybenzamide, G 0639, Urea, 1-((p-(2-(chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-cyclohexyl-, 5-chloro-N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-2-methoxybenzamide, DSSTox_CID_17237, DSSTox_RID_79313, N-(4-(beta-(2-Methoxy-5-chlorbenzamido)-aethyl)-benzolsulfonyl)-N'-cyclohexyl-harnstoff, DSSTox_GSID_37237, U-26452, Benzamide, 5-chloro-N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-2-methoxy-, W-108874, 1-[[p-[2-(5-Chloro-o-anisamido)ethyl]phenyl]sulfonyl]-3-cyclohexylurea, N-p-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulfonyl-N'-cyclohexylurea, 5-chloro-N-(2-{4-[(cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-2-methoxybenzamide, 5-chloro-N-(2-{4-[N-(N-cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-2-methoxybenzamide, 1-{4-[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulfonyl}-3-cyclohexylurea;1-{4-[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulfonyl}-3-cyclohexylurea, GIBENCLAMIDE, SR-01000000196, Glyburide [USAN:USP], glicuformine, Delmide, HB419, HB420, Euglucon (TN), Glibenclamide,(S), 5-Chloro-N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-2-methoxybenzamide, GBM, Prestwick_569, Glibenclamide B.P., Daonil (TN), Glyburide (Diabeta), HB 420, Semi-Daonil (TN), Glibenclamide [INN], RP-1127, Spectrum_000250, Tocris-0911, Opera_ID_801, Prestwick0_000316, Prestwick1_000316, Prestwick2_000316, Prestwick3_000316, Spectrum2_001816, Spectrum3_001327, Spectrum4_001199, Spectrum5_001631, Lopac-G-0639, ACMC-20a66i, D05LYX, Probes1_000431, Probes2_000378, UPCMLD-DP006, AC1L1G1Q, Glibenclamide (JP15/INN), Glibenclamide (JP17/INN), CBiol_001790, Lopac0_000499, Oprea1_764617, SCHEMBL22009, BSPBio_000312, BSPBio_001351, BSPBio_003053, KBioGR_000071, KBioGR_001897, KBioSS_000071, KBioSS_000730, MLS001077262, BIDD:GT0239, DivK1c_000481, SPECTRUM2300229, SPBio_001831, SPBio_002531, AC1Q44V7, BPBio1_000344, GTPL2414, DTXSID0037237, UPCMLD-DP006:001, CTK8B7917, HMS501I03, KBio1_000481, KBio2_000071, KBio2_000730, KBio2_002639, KBio2_003298, KBio2_005207, KBio2_005866, KBio3_000141, KBio3_000142, KBio3_002273, AOB6214, SYN3026, Glybenclamide, >=99% (HPLC), MolPort-000-784-850, NINDS_000481, ZNNLBTZKUZBEKO-UHFFFAOYSA-N, Bio1_000076, Bio1_000565, Bio1_001054, Bio2_000071, Bio2_000551, HMS1361D13, HMS1568P14, HMS1791D13, HMS1922L08, HMS1989D13, HMS2089L06, HMS2093P04, HMS2095P14, HMS3259O12, HMS3261D19, HMS3267A15, HMS3402D13, HMS3428D15, HMS3651E17, HMS3712P14, Pharmakon1600-02300229, ZINC537805, BCP05327, Tox21_110158, Tox21_300758, Tox21_500499, ANW-58936, BDBM50012957, BG0207, CCG-39618, GP8701, HD 419, MFCD00056625, NSC759618, SBB057426, STK362992, AKOS001487495, Tox21_110158_1, BCP9000729, CS-1075, DB01016, KS-5326, LP00499, MCULE-2351642942, NC00566, NSC-759618, IDI1_000481, IDI1_033821, Glibenclamide 1.0 mg/ml in Acetonitrile, NCGC00015467-01, NCGC00015467-02, NCGC00015467-03, NCGC00015467-04, NCGC00015467-05, NCGC00015467-06, NCGC00015467-07, NCGC00015467-08, NCGC00015467-09, NCGC00015467-10, NCGC00015467-12, NCGC00015467-13, NCGC00015467-14, NCGC00015467-15, NCGC00015467-16, NCGC00015467-17, NCGC00015467-18, NCGC00015467-20, NCGC00015467-36, NCGC00016689-01, NCGC00023447-02, NCGC00023447-04, NCGC00023447-05, NCGC00023447-06, NCGC00023447-07, NCGC00023447-08, NCGC00023447-09, NCGC00023447-10, NCGC00023447-11, NCGC00023447-12, NCGC00254662-01, NCGC00261184-01, 5-Chloro-N-[2-[4-[[[(cyclohexylamino)carbonyl]-amino]sulfonyl]phenyl]-ethyl]-2-methoxybenzamide, AJ-23371, AN-20367, CPD000058229, HY-15206, KB-73471, SAM002564212, SC-17437, ST024780, SBI-0050483.P003, AB0012611, AB2000243, Glyburide, meets USP testing specifications, LS-159295, RT-000122, AB00051949, EU-0100499, FT-0601608, G0382, S1716, A19539, C07022, D00336, J10021, W-5054, 78637-EP2270001A1, 78637-EP2270011A1, 78637-EP2272825A2, 78637-EP2272841A1, 78637-EP2275414A1, 78637-EP2287165A2, 78637-EP2287166A2, 78637-EP2292620A2, 78637-EP2298750A1, 78637-EP2298776A1, 78637-EP2298779A1, 78637-EP2301923A1, 78637-EP2301936A1, 78637-EP2305648A1, 78637-EP2308847A1, AB00051949-16, AB00051949-17, AB00051949_18, AB00051949_19, C-22898, I06-0716, SR-01000000196-2, SR-01000000196-4, SR-01000000196-5, SR-01000000196-6, SR-01000000196-8, U-26,452, BRD-K36927236-001-06-0, BRD-K36927236-001-17-7, Z277540138, Glybenclamide, European Pharmacopoeia (EP) Reference Standard, Glyburide, United States Pharmacopeia (USP) Reference Standard, 1-((p-(2-(5-Chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-cyclohexyl urea, 5-Chloro-N-[4-(3-cyclohexylureidosulfonyl)phenethyl]-2-methoxybenzamide, 5-Chloro-N-(4-(N-(cyclohexylcarbamoyl)sulfamoyl)-phenethyl)-2-methoxybenzamide, 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxy-benzamide, 5-chloro-N-[4-({[(cyclohexylamino)carbonyl]amino}sulfonyl)phenethyl]-2-methoxybenzamide, Glibenclamide for peak identification, European Pharmacopoeia (EP) Reference Standard, Glyburide (Glibenclamide), Pharmaceutical Secondary Standard; Certified Reference Material, N-(4-(.beta.-(2-Methoxy-5-chlorbenzamido)-aethyl)-benzolsulfonyl)-N'-cyclohexyl-harnstoff, N-p-[2-(5-Chloro-2-methoxybenzamido)-ethyl]benzene-sulfonyl-N -cyclohexylurea, N-p-[2-(5-Chloro-2-methoxybenzamido)-ethyl]benzene-sulfonyl-N'-cyclohexylurea, N-p-[2-(5-Chloro-2-methoxybenzamido)-ethyl]benzene-sulfonyl-N-cyclohexylurea, (5-chloro-2-methoxyphenyl)-N-[2-(4-{[(cyclohexylamino)carbonylamino]sulfonyl}p henyl)ethyl]carboxamide, 5-Chloro-N-(2-[4-(([(cyclohexylamino)carbonyl]amino)sulfonyl)phenyl]ethyl)-2-methoxybenzamide #, 5-Chloro-N-[2-[4-[[[(Cylcohexylamino)carbonyl]amino]sulphonyl]phenyl]ethyl]-2-methoxybenzamide, N-[(4-{2-[(5-chloro-2-methoxyphenyl)carbonylamino]ethyl}phenyl)sulfonyl](cyclo hexylamino)carboxamide
ID: 38
InChIKey: HKQYGTCOTHHOMP-UHFFFAOYSA-N SMILES: COC1=CC=C(C=C1)C2=COC3=C(C2=O)C=CC(=C3)O
biological descriptors:
CFTR relevance: unspecifiedCategory:
Influence on CFTR function unknown
Order of interaction unknown
subcellular compartment unknown
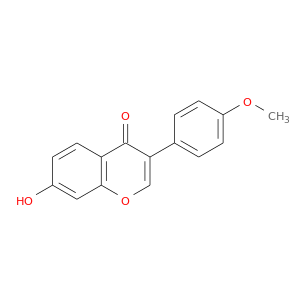
CID is 5280378
synonyms found at PubChem are:
formononetin, 485-72-3, Biochanin B, Formononetol, 7-hydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one, 7-Hydroxy-4'-methoxyisoflavone, 4'-O-methyldaidzein, 7-hydroxy-3-(4-methoxyphenyl)chromen-4-one, 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-methoxyphenyl)-, UNII-295DQC67BJ, 7-Hydroxy-3-(4-methoxyphenyl)-4-benzopyrone, CHEBI:18088, Isoflavone, 7-hydroxy-4'-methoxy-, EINECS 207-623-9, NSC 93360, NSC-93360, 7-Hydroxy-3-(4-methoxyphenyl)chromone, 295DQC67BJ, HKQYGTCOTHHOMP-UHFFFAOYSA-N, NSC93360, IN1330, 7-hydroxy-3-(4-methoxyphenyl)-4H-benzopyran-4-one, Q-100540, 7-Hydroxy-3-(4′-methoxyphenyl)-4H-benzopyran-4-one, SMR000470932, SR-01000765510, formononetine, 7-hydroxy-4'-methoxy-isoflavone, Formononetin,(S), PubChem9850, AC1NQWYT, Spectrum_000373, SpecPlus_000223, Daidzein 4-methyl ether, Spectrum2_000560, Spectrum3_000660, Spectrum4_001429, Spectrum5_000258, DSSTox_CID_2311, D0M7BR, Formononetin (Formononetol), DSSTox_RID_76544, NCIOpen2_005983, DSSTox_GSID_22311, Oprea1_139748, Oprea1_815287, SCHEMBL62915, BSPBio_002299, KBioGR_001878, KBioSS_000853, SPECTRUM102007, MLS000697593, MLS006011897, BIDD:ER0119, DivK1c_006319, SPBio_000639, CHEMBL242341, DTXSID4022311, CTK8C4788, Formononetin, analytical standard, KBio1_001263, KBio2_000853, KBio2_003421, KBio2_005989, KBio3_001519, KS-00000HZV, MolPort-000-450-946, HMS1922N18, HMS2231I04, HMS3369C07, HMS3655N22, ALBB-030789, Formononetin, >=99.0% (TLC), HY-N0183, TNP00176, Tox21_301848, ANW-73131, BBL010458, BDBM50021398, CCG-38727, CF0045, LMPK12050037, MFCD00016948, s2299, SBB016445, STK801612, ZINC18847036, AKOS000270811, AC-8001, AN-8422, CS-3081, MCULE-4171151967, SDCCGMLS-0066428.P001, 7-hydroxy-4'-methoxy-Isoflavone (8CI), NCGC00017269-01, NCGC00017269-02, NCGC00017269-03, NCGC00017269-04, NCGC00017269-05, NCGC00017269-06, NCGC00095207-01, NCGC00095207-02, NCGC00095207-03, NCGC00178715-01, NCGC00255167-01, 4CN-0880, AJ-70674, AK106172, AS-11642, BC216233, CAS-485-72-3, CC-28828, LS-39701, NCI60_042081, SC-04770, ST024713, AB0010475, AX8016260, Isoflavone, 7-hydroxy-4'-methoxy- (8CI), Neochanin; Flavosil;NEOCHANIN;Formononetol, F0868, FT-0626540, FT-0632204, K-080, N1625, 3-(4-methoxyphenyl)-7-oxidanyl-chromen-4-one, C00858, AB00052676-07, 485F723, A827555, AE-641/01968055, C-16842, 7-hydroxy-3-(4-methoxyphenyl)-1-benzopyran-4-one, I06-0241, SR-01000765510-3, SR-01000765510-4, BRD-K55567017-001-02-4, BRD-K55567017-001-06-5, F3139-1207, 7-hydroxy-3-(4-methoxyphenyl)-4H-benzopyran-4-one(9CI), 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-methoxyphenyl)- (9CI), Formononetin, United States Pharmacopeia (USP) Reference Standard, 7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one, 7-Hydroxy-3-(4-methoxyphenyl)chromone, 7-Hydroxy-4'-methoxyisoflavone
ID: 78
InChIKey: WUADCCWRTIWANL-UHFFFAOYSA-N SMILES: COC1=CC=C(C=C1)C2=COC3=CC(=CC(=C3C2=O)O)O
biological descriptors:
CFTR relevance: ΔF508 CFTR activationCategory:
Influence on CFTR function enhances CFTR function
Order of interaction unknown
subcellular compartment unknown
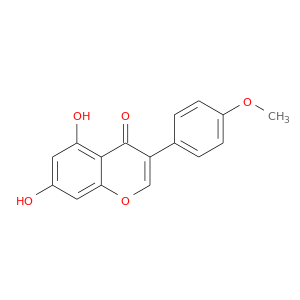
CID is 5280373
synonyms found at PubChem are:
biochanin A, 491-80-5, Biochanin, 4'-Methylgenistein, 5,7-Dihydroxy-4'-methoxyisoflavone, 5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one, Genistein 4-methyl ether, Pratensol, Biochanine A, 5,7-Dihydrox -4'-methoxyisoflavone, 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)-, olmelin, Biochanin-A, 5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one, 4-Methylgenistein, C16H12O5, NSC 123538, UNII-U13J6U390T, CCRIS 5449, 5,7-Dihydroxy-3-p-methoxyphenyl-4H-chromen-4-one, EINECS 207-744-7, NSC123538, Genistein 4'-methyl ether, Isoflavone, 5,7-dihydroxy-4'-methoxy-, MLS000069443, CHEMBL131921, CHEBI:17574, WUADCCWRTIWANL-UHFFFAOYSA-N, U13J6U390T, 4'-Methoxy-5,7-dihydroxy isoflavone, NSC-123538, SMR000059116, Apigenin 4-Methyl Ether, DSSTox_CID_2394, DSSTox_RID_76570, DSSTox_GSID_22394, Q-100552, CAS-491-80-5, SR-01000003021, QSO, Biochanin A, 9, Biochanin A (BCA), AC1NQWYN, Spectrum_000195, Opera_ID_621, Spectrum2_000047, Spectrum3_001098, Spectrum4_001927, Spectrum5_001624, D02UQX, D0S9YX, Oprea1_038096, SCHEMBL61258, BSPBio_002776, KBioGR_002274, KBioSS_000675, MLS001148446, MLS006011785, BIDD:ER0123, DivK1c_001027, SPBio_000173, BDBM9461, GTPL2829, DTXSID1022394, SPECTRUM10100003, Biochanin A (4-Methylgenistein), Biochanin A - 4-Methylgenistein, cid_5280373, HMS503M15, KBio1_001027, KBio2_000675, KBio2_003243, KBio2_005811, KBio3_001996, 5,7-dihydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one, MolPort-000-424-557, NINDS_001027, HMS2232N19, HMS3369A02, HMS3656A13, TNP00319, Isoflavone,7-dihydroxy-4'-methoxy-, Tox21_202097, Tox21_302901, 5,7-Dihydrox-4''-methoxyisoflavone, BBL010523, CCG-38351, LMPK12050229, MFCD00006839, s2377, STK888295, ZINC18847037, 5,7-dihydroxy-4'-methoxy-Isoflavone, 5,7-Dihydroxy-4/'-methoxyisoflavone, AKOS002163860, API0001717, CS-3082, MCULE-6764919720, IDI1_001027, KS-00000I00, SMP1_000045, NCGC00017369-01, NCGC00017369-02, NCGC00017369-03, NCGC00017369-04, NCGC00017369-05, NCGC00017369-06, NCGC00017369-07, NCGC00017369-08, NCGC00017369-09, NCGC00017369-10, NCGC00022428-03, NCGC00022428-04, NCGC00022428-05, NCGC00178478-01, NCGC00256458-01, NCGC00259646-01, AC-22309, AJ-70675, AK155884, AN-45276, AS-17474, HY-14595, KB-79569, LS-39601, NCI60_000558, ST057580, Biochanin A, analytical reference material, AB1004489, AX8016263, B4098, FT-0663120, N1308, V0303, 5,7-dihydroxy-4'-methoxy-Isoflavone (8CI), C00814, X-2593, Isoflavone, 5,7-dihydroxy-4'-methoxy- (8CI), 491B805, 5,7-Dihydroxy-4'-methoxyisoflavone, 98% 250mg, SR-01000003021-4, SR-01000003021-5, BRD-K73303757-001-02-6, BRD-K73303757-001-12-5, I14-13389, 5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one #, F1190-0491, 4H-1-Benzopyran-4-one,7-dihydroxy-3-(4-methoxyphenyl)-, 2AA2D226-B323-4AE2-B576-2D47D15F9845, Biochanin A, United States Pharmacopeia (USP) Reference Standard, 5,7-Dihydroxy-4 inverted exclamation marka-methoxyisoflavone; Genistein 4 inverted exclamation marka-methyl ether